Abstract
Background
Febrile neutropenia (FN) is one of the most common and most critical adverse effects of chemotherapy. Despite many existing guidelines based on the use of granulocyte-colony stimulating factor (G-CSF), FN continues to impair the quality of life and interfere with the treatment of many patients. The purpose of this study was to assess the incidence and management of FN associated with chemotherapy for early breast cancer in routine clinical practice.
Methods
All patients with early-stage breast cancer (ESBC) treated by chemotherapy at Institut Curie, Hôpital René Huguenin, in 2014 were retrospectively included. The incidence and management of FN were reported. Risk factors associated with FN were studied by robust-error-variance Poisson regression.
Results
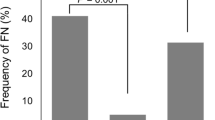
A total of 524 patients received either neoadjuvant (N = 130) or adjuvant chemotherapy (N = 394). Most patients (80%) were treated with a combination of 5-fluorouracil, epirubicin, and cyclophosphamide (FEC100; 3 cycles) followed by docetaxel 100 mg/m2 (D; 3 cycles). The overall incidence of FN was 17%. Eighteen percent of patients received primary prophylaxis (PP) for FN with G-CSF, using pegfilgrastim in 64% of cases and 74% of patients over the age of 70 received PP. Less than 5% of patients who received PP experienced FN. Recurrent FN after secondary prophylaxis was observed in 9% of patients. Forty-seven percent of cases of FN occurred after the first cycle and 30% occurred after the fourth cycle, corresponding to D ± trastuzumab (T). The FEC100 regimen was associated with a relative risk of FN of 1.98 (p = 0.09). Autoimmune (AI) and inflammatory diseases were associated with a higher risk of FN (RR 3.08; p < 0.01). No significant difference in the incidence of FN was observed between adjuvant and neoadjuvant chemotherapy. FN was managed on an outpatient basis in 72% of cases. Outpatients with FN were mainly treated by a combination of amoxicillin–clavulanic acid and ciprofloxacin. Dose reduction or chemotherapy regimen modification were necessary in 25% of patients after FN. No toxic death was reported.
Conclusion
The incidence of FN induced by adjuvant/neoadjuvant chemotherapy in ESBC is higher in routine clinical practice than in clinical trials. AI or inflammatory diseases were significant independent risk factors for FN. Primary prophylaxis in patients at risk (elderly, comorbid patients), especially treated with the FEC regimen, is the keystone of management of this adverse effect. Prevention and management of FN to ensure the patient’s safety and quality of life are a major issue for both medical oncologists and supportive care physicians.

Similar content being viewed by others
References
Les cancers en France, édition2016, INCa 2017, http://www.e-cancer.fr/ressources/cancers_en_france/#page=13
Kuderer NM (2006) Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer 106:2258–2266
Lyman GH, Rolston (2010) How we treat febrile neutropenia in patients receiving cancer chemotherapy. J Oncol Pract 6(3):149–152
Kosaka Y (2015) Phase III placebo-controlled, double-blind, randomized trial of pegfilgrastim to reduce the risk of febrile neutropenia in breast cancer patients receiving docetaxel/cyclophosphamide chemotherapy. Support Care Cancer 23(4):1137–1143
Smith TJ et al (2006) Update recommendations for the use of white blood cell growth factors: an evidence based clinical practice guideline. J Clinic Oncol 24(19):3187–3205
Aapro MS et al (2011) 2010 update of EORTC guidelines for the use of GCSF to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumors. Eur J Cancer 47(1):8–32
Klastersky J et al (2016) Management of febrile neutropaenia : ESMO clinical practice guidelines. Ann Oncol 27(Supplement 5):v111–v118
Roche H et al (2006) Sequential adjuvant epirubicin-based and docetaxel chemotherapy for node-positive breast cancer patients: the FNCLCC PACS 01 trial. J Clin Oncol 24:5664–5671
Cousin S et al (2012) Febrile neutropenia incidence and hematological toxicity with the FEC100- docetaxel regimen in the treatment of early-stage breast cancer. Bull Cancer 99(7–8):75–80
Rayson D et al (2012) Incidence of febrile neutropenia during adjuvant chemotherapy for breast cancer: a prospective study. Curr Oncol 19(3):e216–e218
Younis T et al (2012) Primary G-CSF prophylaxis for adjuvant TC or FEC-D chemotherapy outside of clinical trial settings: a systemic review and meta-analysis. Support Care Cancer 20(10):2523–2530
Assi H et al (2014) Incidence of febrile neutropenia in early stage breast cancer patients receiving adjuvant FEC-D treatment. Support Care Cancer 22:3227–3234
Miguel I, Winckler P, Sousa M, Cardoso C, Moreira A, Brito M (2015) Febrile neutropenia in FEC-D regimen for early stage breast cancer: is there a place for G-CSF primary prophylaxis? Breast Dis 35(3):167–171
Hughes WT, Armstrong D, Bodey GP et al (2002) Guidelines for the use of antimicrobial agents in neutropenic patients with cancer. Clin Infect Dis 34:730–751
Zou G (2004) A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 159(7):702–706
Jacot W (2015) Granulocyte-colony stimulating factor (G-CSF) use in clinical practice in patients receiving chemotherapy for breast cancer: the Opaline Study. Bull Cancer 102(12):979–992
Maenpaa J (2016) The use of granulocyte colony stimulating factor (G-CSF) and management of chemotherapy delivery during adjuvant treatment for early-stage breast cancer: further observations from the IMPACT solid study. Breast 25:27–33
Kern WV (2013) Oral antibiotics for fever in low-risk neutropenic patients with cancer : a double-blind, randomized, multicenter trial comparing single daily moxifloxacin with twice daily ciprofloxacin plus amoxicillin/clavulanic acid combination therapy—EORTC infectious diseases group trial XV. JCO 31(9):1149–1156
Weycker D (2012) Risk and healthcare costs of chemotherapy-induced neutropenic complications in women with metastatic breast cancer. Chemotherapy 58(1):8–18
Rouge-Bugat ME (2015) Guideline sheets on the side effects of anticancer drugs are useful for general practitioners. Support Care Cancer 23(12):3473–3480
Madarnas Y (2011) Real-world experience with adjuvant FEC-D chemotherapy in four Ontario regional cancer centers. Curr Oncol 18(3):119–125
Raza S (2009) Relative dose intensity delivered to patients with early breast cancer: Canadian experience. Curr Oncol 16(6):8–12
Calip GS (2015) Myelodysplastic syndrome and acute myeloid leukemia following adjuvant chemotherapy with and without granulocyte colony-stimulating factors for breast cancer. Breast Cancer Res Treat 154:133–143
Li X, Luthra R, Morrow PK, Fisher MD, Reiner M, Barron RL, Langeberg WJ (2016) Comorbidities among patients with cancer who do and do not develop febrile neutropenia during the first chemotherapy cycle. J Oncol Pharm Pract 22(5):679–689
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
They have full control of all primary data and agree to allow the journal to review their data if requested.
Rights and permissions
About this article
Cite this article
Bacrie, J., Laurans, M., Iorio, P. et al. Febrile neutropenia in adjuvant and neoadjuvant chemotherapy for breast cancer: a retrospective study in routine clinical practice from a single institution. Support Care Cancer 26, 4097–4103 (2018). https://doi.org/10.1007/s00520-018-4280-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-018-4280-4