Abstract
Purpose
Malignant ascites is one of the symptoms causing discomfort in advanced cancer patients. Cell-free and concentrated ascites reinfusion therapy (CART) is one treatment modality, but controlled trials are limited. The primary aim of this study was to explore the efficacy and safety of CART, as well as their predictors, to obtain data for planning a further controlled trial.
Methods
This was a single center retrospective cohort study in patients with refractory malignant ascites. Consecutive adult patients who underwent CART were enrolled. The primary endpoints were the time to next paracentesis and seven patient-reported symptoms (e.g., abdominal pain and distension). The secondary endpoints were adverse events, laboratory findings, and physical findings.
Results
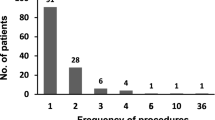
A total of 104 CART procedures for 51 patients were analyzed. The median time to next paracentesis was 27 days (95% CI, 21–35). Intensities of all seven symptoms were significantly improved after CART (p < 0.0001 for all symptoms). Grade 3 hypotension occurred during one procedure, and mild fever occurred in 5%. Total protein, albumin, and estimated glomerular filtration rate were significantly increased. Hemorrhagic ascites, ascites white blood cell count, serum total protein, and lymphocyte percentages were the independent predictors of the time to next paracentesis.
Conclusion
The effects of reinfusion of concentrated ascitic fluid may be maintained for 1 month, being potentially longer than that of total paracentesis alone. This study had no comparison groups and examined the short-term effect. A randomized controlled study to compare the long-term effects of total paracentesis alone vs. CART is necessary.

Similar content being viewed by others
References
Smith EM, Jayson GC (2003) The current and future management of malignant ascites. Clin Oncol (R Coll Radiol) 15(2):59–72. https://doi.org/10.1053/clon.2002.0135
Keen J (2015) Jaundice, ascites, and encephalopathy. In: Cherny NI, Fallon MT, Kaasa S, Portenoy RK, Currow DC (eds) Oxford textbook of palliative medicine, 5th edn. Oxford University Press, New York, pp 686–701
Becker G, Galandi D, Blum HE (2006) Malignant ascites: systematic review and guideline for treatment. Eur J Cancer 42(5):589–597. https://doi.org/10.1016/j.ejca.2005.11.018
Cavazzoni E, Bugiantella W, Graziosi L, Franceschini MS, Donini A (2013) Malignant ascites: pathophysiology and treatment. Int J Clin Oncol 18(1):1–9. https://doi.org/10.1007/s10147-012-0396-6
Runyon BA (1994) Care of patients with ascites. N Engl J Med 330(5):337–342. https://doi.org/10.1056/NEJM199402033300508
Levy VG, Hecht Y, Georgacopoulos H, Hadchouel P, Touboul JP, Sausse A et al (1971) Drying of cirrhotic ascites by continuous intravenous reinjection of concentrated ascitic fluid. Ann Med Interne (Paris) 122(11):1075–1085
Parbhoo SP, Ajdukiewicz A, Sherlock S (1974) Treatment of ascites by continuous ultrafiltration and reinfusion of protein concentrate. Lancet 1(7864):949–952
Smart HL, Triger DR (1990) A randomised prospective trial comparing daily paracentesis and intravenous albumin with recirculation in diuretic refractory ascites. J Hepatol 10(2):191–197. https://doi.org/10.1016/0168-8278(90)90051-R
Bruno S, Borzio M, Romagnoni M, Battezzati PM, Rossi S, Chiesa A, Podda M (1992) Comparison of spontaneous ascites filtration and reinfusion with total paracentesis with intravenous albumin infusion in cirrhotic patients with tense ascites. BMJ 304(6843):1655–1658. https://doi.org/10.1136/bmj.304.6843.1655
Graziotto A, Rossaro L, Inturri P, Salvagnini M (1997) Reinfusion of concentrated ascitic fluid versus total paracentesis. A randomized prospective trial. Dig Dis Sci 42(8):1708–1714. https://doi.org/10.1023/A:1018865516168
Landini S, Coli U, Fracasso A, Morachiello P, Righetto F, Scanferla F, Genchi R, Bazzato G (1985) Spontaneous ascites filtration and reinfusion (SAFR) in cirrhotic patients. Int J Artif Organs 8(5):277–280
Villeneuve JP, Thuot C, Marleau D, Joly JG, Huet PM, Viallet A (1977) Treatment of resistant ascites by continuous ultrafiltration—reinfusion of ascitic fluid. Can Med Assoc J 117(11):1296–1298
Inoue N, Yamazaki Z, Oda T, Sugiura M, Wada T (1977) Treatment of intractable ascites by continuous reinfusion of the sterilized, cell-free and concentrated ascitic fluid. Trans Am Soc Artif Intern Organs 23:699–702
Ito T, Hanafusa N, Iwase S, Noiri E, Nangaku M, Nakagawa K, Miyagawa K (2015) Effects of cell-free and concentrated ascites reinfusion therapy (CART) on symptom relief of malignancy-related ascites. Int J Clin Oncol 20(3):623–628. https://doi.org/10.1007/s10147-014-0750-y
Ito T, Hanafusa N, Fukui M, Yamamoto H, Watanabe Y, Noiri E, Iwase S, Miyagawa K, Fujita T, Nangaku M (2014) Single center experience of cell-free and concentrated ascites reinfusion therapy in malignancy related ascites. Ther Apher Dial 18(1):87–92. https://doi.org/10.1111/1744-9987.12049
Yamaguchi H, Kitayama J, Emoto S, Ishigami H, Ito T, Hanafusa N, Watanabe T (2015) Cell-free and concentrated ascites reinfusion therapy (CART) for management of massive malignant ascites in gastric cancer patients with peritoneal metastasis treated with intravenous and intraperitoneal paclitaxel with oral S-1. Eur J Surg Oncol 41(7):875–880. https://doi.org/10.1016/j.ejso.2015.04.013
Katoh S, Kojima T, Itoh K, Yoneyama S, Ida K, Nakaji S (1997) Usefulness of a nonmachinery based system for the reinfusion of cell-free and concentrated autogenous ascitic fluid. Artif Organs 21(12):1232–1238
Katoh S, Tatsukawa H, Kondoh M, Inoue M, Ida K, Miyagawa F (1991) Prevention of the febrile reaction occurring on reinfusion of cell-free and concentrated autogenous ascites. Jpn J Med 30(4):311–317. https://doi.org/10.2169/internalmedicine1962.30.311
Orimi S, Mizuno K, Narahara M, Umakosi H, Kaihara M, Hashimoto M (2011) A study of appropriate flow rate settings for cell-free and concentrated ascites reinfusion therapy and change of cytokine concentrations in ascites. Ther Apher Dial 15(4):411–414. https://doi.org/10.1111/j.1744-9987.2011.00973.x
Maeda O, Ando T, Ishiguro K, Watanabe O, Miyahara R, Nakamura M, Funasaka K, Kazuhiro F, Ando Y, Goto H (2014) Safety of repeated cell-free and concentrated ascites reinfusion therapy for malignant ascites from gastrointestinal cancer. Mol Clin Oncol 2(6):1103–1106. https://doi.org/10.3892/mco.2014.335
Japanese CSG, Matsusaki K, Ohta K, Yoshizawa A, Gyoda Y (2011) Novel cell-free and concentrated ascites reinfusion therapy (KM-CART) for refractory ascites associated with cancerous peritonitis: its effect and future perspectives. Int J Clin Oncol 16:395–400
Heiss MM, Murawa P, Koralewski P, Kutarska E, Kolesnik OO, Ivanchenko VV, Dudnichenko AS, Aleknaviciene B, Razbadauskas A, Gore M, Ganea-Motan E, Ciuleanu T, Wimberger P, Schmittel A, Schmalfeldt B, Burges A, Bokemeyer C, Lindhofer H, Lahr A, Parsons SL (2010) The trifunctional antibody catumaxomab for the treatment of malignant ascites due to epithelial cancer: results of a prospective randomized phase II/III trial. Int J Cancer 127(9):2209–2221. https://doi.org/10.1002/ijc.25423
Jatoi A, Nieva JJ, Qin R, Loprinzi CL, Wos EJ, Novotny PJ, Moore DF Jr, Mowat RB, Bechar N, Pajon ER Jr, Hartmann LC (2012) A pilot study of long-acting octreotide for symptomatic malignant ascites. Oncology 82(6):315–320. https://doi.org/10.1159/000337246
Ross GJ, Kessler HB, Clair MR, Gatenby RA, Hartz WH, Ross LV (1989) Sonographically guided paracentesis for palliation of symptomatic malignant ascites. AJR Am J Roentgenol 153(6):1309–1311. https://doi.org/10.2214/ajr.153.6.1309
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A, Collaborators developing the Japanese equation for estimated GFR (2009) Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 53(6):982–992. https://doi.org/10.1053/j.ajkd.2008.12.034
Morita T, Tsunoda J, Inoue S, Chihara S (1999) The palliative prognostic index: a scoring system for survival prediction of terminally ill cancer patients. Support Care Cancer 7(3):128–133. https://doi.org/10.1007/s005200050242
Yamaguchi T, Mori T, Takahashi K, Matsumoto H, Miyamoto H, Kato T (2008) A new classification system for liver metastases from colorectal cancer in Japanese multicenter analysis. Hepato-Gastroenterology 55(81):173–178
Anthony T, Baron T, Mercadante S, Green S, Chi D, Cunningham J, Herbst A, Smart E, Krouse RS (2007) Report of the clinical protocol committee: development of randomized trials for malignant bowel obstruction. J Pain Symptom Manag 34(1):S49–S59. https://doi.org/10.1016/j.jpainsymman.2007.04.011
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48(3):452–458. https://doi.org/10.1038/bmt.2012.244
Easson AM, Bezjak A, Ross S, Wright JG (2007) The ability of existing questionnaires to measure symptom change after paracentesis for symptomatic ascites. Ann Surg Oncol 14(8):2348–2357. https://doi.org/10.1245/s10434-007-9370-3
Runyon BA, Hoefs JC, Morgan TR (1988) Ascitic fluid analysis in malignancy-related ascites. Hepatology 8(5):1104–1109. https://doi.org/10.1002/hep.1840080521
Urrunaga NH, Singal AG, Cuthbert JA, Rockey DC (2013) Hemorrhagic ascites. Clinical presentation and outcomes in patients with cirrhosis. J Hepatol 58(6):1113–1118. https://doi.org/10.1016/j.jhep.2013.01.015
Funding
This study was supported by Japan Society for the Promotion of Science KAKENHI Grant Number JP16H05212.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved by the institutional review board of Nissay General Hospital.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOCX 147 kb)
Rights and permissions
About this article
Cite this article
Hanada, R., Yokomichi, N., Kato, C. et al. Efficacy and safety of reinfusion of concentrated ascitic fluid for malignant ascites: a concept-proof study. Support Care Cancer 26, 1489–1497 (2018). https://doi.org/10.1007/s00520-017-3980-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-017-3980-5