Abstract
Purpose
Chemotherapy-induced nausea and vomiting (CINV) causes significant morbidity among colorectal cancer patients, receiving fluorouracil, oxaliplatin, and leucovorin (FOLFOX) chemotherapy even with standard antiemetic prophylaxis. The purpose of this study is to determine if the addition of aprepitant to standard antiemetic therapy improves CINV in these patients.
Methods
Patients receiving FOLFOX for colorectal cancer were given antiemetic prophylaxis with aprepitant 125 mg orally on day 1 and 80 mg on days 2 and 3. Palonosetron 0.25 mg was given IV push on day 1 only. Dexamethasone 12 mg was administered orally on day 1 and 8 mg each morning on days 2 through 4. Assessments including emetic events, rescue doses, nutritional intake, and appetite were recorded in a patient diary which was returned to study personnel in the following cycle.
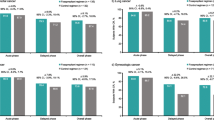
Results
Of the 53 patients screened, 50 were evaluable and had a complete dataset for cycle 1. For the first cycle, 74% of patients achieved a complete response (CR), 22% achieved a major response and 4% experienced treatment failure. The percentage of patients achieving a CR remained high throughout each cycle at 83, 83, and 86% for cycles 2, 3, and 4, respectively. Appetite and nutritional status remained largely unchanged throughout treatment. Adverse events occurring in more than 10% of patients included diarrhea (13.6%), fatigue (12.6%), and neutropenia (11%).
Conclusions
Aprepitant added to standard antiemetic therapy appears to be an effective and safe regimen for prevention of CINV in patients receiving FOLFOX.
Similar content being viewed by others
References
André T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan P, Bridgewater J, Tabah-Fisch I, de Gramont A (2004) Multicenter International Study of Oxaliplatin/5-Fluorouracil/Leucovorin in the Adjuvant Treatment of Colon Cancer (MOSAIC) Investigators. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 350(23):2343–2351
Sommariva S, Pongiglione B, Tarricone R (2016) Impact of chemotherapy-induced nausea and vomiting on health-related quality of life and resource utilization: a systematic review. Crit Rev Oncol Hematol 99:13–36
Falcone A, Ricci S, Brunetti I, Pfanner E, Allegrini G, Barbara C, Crinò L, Benedetti G, Evangelista W, Fanchini L, Cortesi E, Picone V, Vitello S, Chiara S, Granetto C, Porcile G, Fioretto L, Orlandini C, Andreuccetti M, Masi G, Ovest GON (2007) Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J Clin Oncol 25(13):1670–1676
Hesketh PJ, Sanz-Altamira P, Bushey J, Hesketh AM (2012) Prospective evaluation of the incidence of delayed nausea and vomiting in patients with colorectal cancer receiving oxaliplatin-based chemotherapy. Support Care Cancer 20(5):1043–1047. https://doi.org/10.1007/s00520-011-1180-2
Poli-Bigelli S, Rodrigues-Pereira J, Carides AD, Julie Ma G, Eldridge K, Hipple A, Evans JK, Horgan KJ, Lawson F (2003) Aprepitant Protocol 054 Study Group. Addition of the neurokinin 1 receptor antagonist aprepitant to standard antiemetic therapy improves control of chemotherapy-induced nausea and vomiting. Results from a randomized, double-blind, placebo-controlled trial in Latin America. Cancer 97(12):3090–3098
Hesketh PJ, Grunberg SM, Gralla RJ, Warr DG, Roila F, de Wit R, Chawla SP, Carides AD, Ianus J, Elmer ME, Evans JK, Beck K, Reines S, Horgan KJ (2003) Aprepitant Protocol 052 Study Group. The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin—the Aprepitant Protocol 052 Study Group. J Clin Oncol 21(22):4112–4119
Grote T, Hajdenberg A, Cartmell S et al (2005) Palonosetron plus aprepitant and dexamethasone is a highly effective combination to prevent chemotherapy-induced nausea and vomiting after emetogenic chemotherapy. Eur J Cancer 3:371 (abstr)
Hesketh PJ (1999) Defining the emetogenicity of cancer chemotherapy regimens: relevance to clinical practice. Oncologist 4(3):191–196
Cancer Therapy Evaluation Program (2006) Common Terminology Criteria for Adverse Events v3.0. DCTD, NCI, NIH, DHHS. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf. Accessed 12/7/16
Takemoto H, Nishimura J, Komori T, Kim HM, Ota H, Suzuki R, Ikenaga M, Ikeda M, Yamamoto H, Satoh T, Hata T, Takemasa I, Mizushima T, Doki Y, Mori M, Multicenter Clinical Study Group of Osaka, Colorectal Cancer Treatment Group (MCSGO) (2016) Combination antiemetic therapy with aprepitant/fosaprepitant in patients with colorectal cancer receiving oxaliplatin-based chemotherapy in the SENRI trial: analysis of risk factors for vomiting and nausea. Int J Clin Oncol 22(1):88–95. https://doi.org/10.1007/s10147-016-1022-9
Conroy T et al (2010) Quality-of-life findings from a randomised phase-III study of XELOX vs FOLFOX-6 in metastatic colorectal cancer. Br J Cancer 102(1):59–67. https://doi.org/10.1038/sj.bjc.6605442
Matsuda M, Yamamoto T, Ishikawa E, Akutsu H, Takano S, Matsumura A (2016) Combination of palonosetron, aprepitant, and dexamethasone effectively controls chemotherapy-induced nausea and vomiting in patients treated with concomitant temozolomide and radiotherapy: results of a prospective study. Neurol Med Chir (Tokyo) 56(11):698–703
National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines) (2016) Antiemesis. National Comprehensive Cancer Network. https://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf. Accessed 12/7/16
Hesketh PJ, Bohlke K, Lyman GH, Basch E, Chesney M, Clark-Snow RA, Danso MA, Jordan K, Somerfield MR, Kris MG, American Society of Clinical Oncology (2016) Antiemetics: American Society of Clinical Oncology focused guideline update. J Clin Oncol 34(4):381–386. https://doi.org/10.1200/JCO.2015.64.3635
Nishimura J et al (2015) Combination antiemetic therapy with aprepitant/fosaprepitant in patients with colorectal cancer receiving oxaliplatin-based chemotherapy (SENRI trial): a multicentre, randomised, controlled phase 3 trial. Eur J Cancer 51(10):1274–1282. https://doi.org/10.1016/j.ejca.2015.03.024
Lexi-Drugs (2017) Aprepitant. Lexicomp. http://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/6376. Accessed 4/11/17
Lexi-Drugs (2017) Fosaprepitant. Lexicomp. http://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/1065839. Accessed 4/20/17
Moore S, Tumeh J, Wojtanowski S, Flowers C (2007) Cost-effectives of aprepitant for the prevention of chemotherapy-induced nausea and vomiting associated with highly emetogenic chemotherapy. Value Health 10(1):23–31
Chan SL, Jen J, Burke T, Pellissier J (2014) Economic analysis of aprepitant-containing regimen to prevent chemotherapy-induced nausea and vomiting in patients receiving highly emetogenic chemotherapy in Hong Kong. Asia Pac J Clin Oncol 10(1):80–91. https://doi.org/10.1111/ajco.12170
Annemans L, Strens D, Lox E, Petit C, Malonne H (2008) Cost-effectiveness analysis of aprepitant in the prevention of chemotherapy-induced nausea and vomiting in Belgium. Support Care Cancer 16(8):905–915
Lordick F, Ehlken B, Ihbe-Heffinger A, Berger K, Krobot KJ, Pellissier J, Davies G, Deuson R (2007) Health outcomes and cost-effectiveness of aprepitant in outpatients receiving antiemetic prophylaxis for highly emetogenic chemotherapy in Germany. Eur J Cancer 43(2):299–307
Abbrederis K, Lorenzen S, Rothling N, Ihbe-Heffinger A, Schuster T, Peschel C, Lordick F (2009) Chemotherapy-induced nausea and vomiting in the treatment of gastrointestinal tumors and secondary prophylaxis with aprepitant. Onkologie 32(1–2):30–34. https://doi.org/10.1159/000183735
CE W, Liaw CC (2012) Using aprepitant as secondary antiemetic prophylaxis for cancer patients with cisplatin-induced emesis. Support Care Cancer 20(10):2357–2361. https://doi.org/10.1007/s00520-011-1345-z
Funding
This study is funded by Merck & Co., Inc., Kenilworth, NJ.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Dr. Bubalo reports grants from Merck during the conduct of the study. All other authors have nothing to disclose.
Conflict of interest
The authors declare that they have no conflict of interest.
Disclosure
All other authors have nothing to disclose.
Additional information
Dr. Edwards was at Walter Reed Army Medical Center when the study was completed.
Rights and permissions
About this article
Cite this article
Bubalo, J.S., Herrington, J.D., Takemoto, M. et al. Phase II open label pilot trial of aprepitant and palonosetron for the prevention of chemotherapy-induced nausea and vomiting (CINV) in patients receiving moderately emetogenic FOLFOX chemotherapy for the treatment of colorectal cancer. Support Care Cancer 26, 1273–1279 (2018). https://doi.org/10.1007/s00520-017-3950-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-017-3950-y