Abstract
Purpose
Identifying risk factors for premature totally implantable venous access device (TIVAD) catheter removal is crucial; however, because of the diversity of study methodologies, there is no consensus on such factors. The objective of the present study was to identify such risk factors by applying a cohort design study with a long-term follow-up period.
Methods
For this cohort study, we selected cancer patients who had newly implanted TIVADs between July 2008 and December 2008. The follow-up period lasted until September 2012. Univariate analysis was performed for age, gender, cancer type, TIVAD brand, puncture site, sidedness of puncture, and catheter tip position. The hazard ratio (HR) of potential risk factors was calculated using the Cox proportional hazards regression model, and Kaplan–Meier curves were applied for catheter survival analysis.
Results
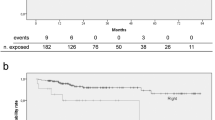
Our study consisted of 240 people, with 5 people lost to follow-up. The cumulative premature catheter removal rate of all TIVADs was 9.8%, with the most common reason for premature removal being port-associated blood stream infection (PABSI), which proved to be highest in patients with hematology cancer (27.8%) and upper gastrointestinal cancer (19.4%). Suboptimal tip position (HR 5.13, 95% confidence interval 1.73–15.21) was also a risk factor for premature removal, and it was correlated with symptomatic TIVAD occlusion (p = 0.0004).
Conclusions
PABSI was the most common reason for premature catheter removal, with a varied incidence rate between different cancer types. Suboptimal tip position was also a risk factor. Confirming the final tip position after implantation is crucial. Infection control is important for TIVAD care, especially in high-risk cancer patients.

Similar content being viewed by others
References
Biffi R, de Braud F, Orsi F, Pozzi S, Mauri S, Goldhirsch A, Nole F, Andreoni B (1998) Totally implantable central venous access ports for long-term chemotherapy. A prospective study analyzing complications and costs of 333 devices with a minimum follow-up of 180 days. Ann Oncol 9:767–773
Cadman A, Lawrance JA, Fitzsimmons L, Spencer-Shaw A, Swindell R (2004) To clot or not to clot? That is the question in central venous catheters. Clinical Radiology 59:349–355
Chang L, Tsai JS, Huang SJ, Shih CC (2003) Evaluation of infectious complications of the implantable venous access system in a general oncologic population. Am J Infect Control 31:34–39
Chen IC, Hsu C, Chen YC, Chien SF, Kao HF, Chang SY, Hu FC, Yeh KH (2013) Predictors of bloodstream infection associated with permanently implantable venous port in solid cancer patients. Ann Oncol 24:463–468
Covey AM, Toro-Pape FW, Thornton RH, Son C, Erinjeri J, Sofocleous CT, Brody LA, Brown KT, Sepkowitz KA, Getrajdman GI (2012) Totally implantable venous access device placement by interventional radiologists: are prophylactic antibiotics necessary? J Vasc Interv Radiol 23:358–362
Hsieh CC, Weng HH, Huang WS, Wang WK, Kao CL, Lu MS, Wang CS (2009) Analysis of risk factors for central venous port failure in cancer patients. World Journal of gastroenterology: WJG 15:4709–4714
Ji L, Yang J, Miao J, Shao Q, Cao Y, Li H (2015) Infections related to totally implantable venous-access ports: long-term experience in one center. Cell Biochem Biophys 72:235–240
Justo JA, Bookstaver PB (2014) Antibiotic lock therapy: review of technique and logistical challenges. Infect Drug Resist 7:343–363
Kao HF, Chen IC, Hsu C, Chang SY, Chien SF, Chen YC, Hu FC, Yang JC, Cheng AL, Yeh KH (2014) Chlorhexidine for the prevention of bloodstream infection associated with totally implantable venous ports in patients with solid cancers. Support Care Cancer 22:1189–1197
Karanlik H, Kurul S (2009) Modification of approach for totally implantable venous access device decreases rate of complications. J Surg Oncol 100:279–283
Karanlik H, Kurul S, Saip P, Unal ES, Sen F, Disci R, Topuz E (2011) The role of antibiotic prophylaxis in totally implantable venous access device placement: results of a single-center prospective randomized trial. Am J Surg 202:10–15
Kim JT, Oh TY, Chang WH, Jeong YK (2012) Clinical review and analysis of complications of totally implantable venous access devices for chemotherapy. Med Oncol 29:1361–1364
Kohr D, Singh P, Tschernatsch M, Kaps M, Pouokam E, Diener M, Kummer W, Birklein F, Vincent A, Goebel A, Wallukat G, Blaes F (2011) Autoimmunity against the beta2 adrenergic receptor and muscarinic-2 receptor in complex regional pain syndrome. Pain 152:2690–2700
Lai NM, Chaiyakunapruk N, Lai NA, O'Riordan E, Pau WS, Saint S (2016) Catheter impregnation, coating or bonding for reducing central venous catheter-related infections in adults. Cochrane Database Syst Rev 3:CD007878
Lebeaux D, Larroque B, Gellen-Dautremer J, Leflon-Guibout V, Dreyer C, Bialek S, Froissart A, Hentic O, Tessier C, Ruimy R, Pelletier AL, Crestani B, Fournier M, Papo T, Barry B, Zarrouk V, Fantin B (2012) Clinical outcome after a totally implantable venous access port-related infection in cancer patients: a prospective study and review of the literature. Medicine (Baltimore) 91:309–318
Lin CH, Wu HS, Chan DC, Hsieh CB, Huang MH, Yu JC (2010) The mechanisms of failure of totally implantable central venous access system: analysis of 73 cases with fracture of catheter. European Journal of Surgical oncology: the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology 36:100–103
Lin CP, Wang YC, Lin FS, Huang CH, Sun WZ (2011) Ultrasound-assisted percutaneous catheterization of the axillary vein for totally implantable venous access device. European Journal of Surgical Oncology: the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology 37:448–451
Nishinari K, Wolosker N, Bernardi CV, Yazbek G (2010) Totally implantable ports connected to valved catheters for chemotherapy: experience from 350 Groshong devices. J Vasc Access 11:17–22
Schutz JC, Patel AA, Clark TW, Solomon JA, Freiman DB, Tuite CM, Mondschein JI, Soulen MC, Shlansky-Goldberg RD, Stavropoulos SW, Kwak A, Chittams JL, Trerotola SO (2004) Relationship between chest port catheter tip position and port malfunction after interventional radiologic placement. J Vasc Interv Radiol 15:581–587
Subramaniam A, Kim KH, Bryant SA, Kimball KJ, Huh WK, Straughn JM, Estes JM, Alvarez RD (2011) Incidence of mechanical malfunction in low-profile subcutaneous implantable venous access devices in patients receiving chemotherapy for gynecologic malignancies. Gynecol Oncol 123:54–57
Taylor JE, Tan K, Lai NM, McDonald SJ (2015) Antibiotic lock for the prevention of catheter-related infection in neonates. Cochrane Database Syst Rev 6:CD010336
Torramade JR, Cienfuegos JA, Hernandez JL, Pardo F, Benito C, Gonzalez J, Balen E, de Villa V (1993) The complications of central venous access systems: a study of 218 patients. Eur J Surg 159:323–327
Tsai YF, Ku YH, Chen SW, Huang WT, Lu CC, Tsao CJ (2012) Right- and left-subclavian vein port-a-cath systems: comparison of complications. Eur Surg Res 49:66–72
van de Wetering MD, van Woensel JBM, Lawrie TA. (2013) Prophylactic antibiotics for preventing Gram positive infections associated with long-term central venous catheters in oncology patients. Cochrane Database of Systematic Reviews. doi:10.1002/14651858.CD003295.pub3
Vandoni RE, Guerra A, Sanna P, Bogen M, Cavalli F, Gertsch P (2009) Randomised comparison of complications from three different permanent central venous access systems. Swiss Med Wkly 139:313–316
Wang TY, Lee KD, Chen PT, Chen MC, Chen YY, Huang CE, Kuan FC, Chen CC, Lu CH (2015) Incidence and risk factors for central venous access port-related infection in Chinese cancer patients. J Formos Med Assoc 114:1055–1060
Wang YC, Huang CH, Lin FS, Lin WY, Fan SZ, Lin CP, Sun WZ (2012) Intravenous electrocardiography helps inexperienced operators to place totally implantable venous access device more accurately. J Surg Oncol 105:848–851
Acknowledgments
The authors acknowledge the statistical assistance provided by the Taiwan Clinical Trial Statistical Center, Training Center, and Pharmacogenomics Laboratory (founded by the National Research Program for Biopharmaceuticals (NRPB) at the Ministry of Science and Technology in Taiwan; MOST 104-2325-B-002-032), as well as the Department of Medical Research at National Taiwan University Hospital.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Wang, YC., Lin, PL., Chou, WH. et al. Long-term outcomes of totally implantable venous access devices. Support Care Cancer 25, 2049–2054 (2017). https://doi.org/10.1007/s00520-017-3592-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-017-3592-0