Abstract
Context
Fatigue is the most common under-recognized symptom in cancer. Administering fatigue tools in multi-lingual and multi-literate populations may affect the quality and accuracy of the data collected as they rely on language to elicit responses.
Aim
The aim of the study is to develop and validate a tool to assess fatigue in cancer patients using response formats that are not language-dependent.
Methods
The content validity of the tool was established using the Delphi procedure and was field tested with 102 cancer patients. Test-retest reliability of the tool was tested with 55 cancer patients and 47 healthy individuals. Convergent, concurrent, and discriminant validity and internal consistency were established with 374 cancer patients, 202 survivors, and 75 healthy controls.
Statistical analysis
Qualitative analyses, descriptive statistics, product-moment correlation, analysis of variance, Cronbach’s α coefficient, and exploratory factor analysis were conducted.
Results
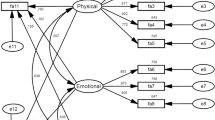
The Cronbach’s alpha of the SAFE in cancer patients and healthy individuals was .86 and .92, and their test-retest reliability ranged from .44 to .83. SAFE correlated significantly with measures of quality of life (QOL) (r = −0.54, p < .01), anxiety (r = 0.54, p < .01), depression (r = 0.5, p < .01), and sleep (r = 0.52, p < .01). The tool was able to distinguish between cancer patients, survivors, and healthy controls (p < .05). Two factors emerged namely “Fatigue Extent and impact” and “General fatigue” contributing to 52% of the variance in fatigue.
Conclusion
A symbolic tool using visual response formats to assess fatigue and its impact in cancer patients was developed and standardized with good reliability and construct, concurrent, and discriminant validity established.

Similar content being viewed by others
References
Lawrence DP, Kupelnick B, Miller K, Devine D, Lau J (2004) Evidence report on the occurence, assessment and treatment of fatigue in cancer patients. Journal of National Cancer Institute Monographs 32:40–50. doi:10.1093/jncimonographs/lgh027
Hofman M, Ryan JL, Figueroa-Moseley CD, Jean-Pierre P, Morrow GR (2007) Cancer-related fatigue: the scale of the problem. Oncologist 12(Suppl 1):4–10. doi:10.1634/theoncologist.12-S1-4
Jean-Pierre P, Figueroa-Moseley CD, Kohli S, Fiscella K, Palesh OG, Morrow GR (2007) Assesement of cancer-related fatigue: implications for clinical diagnosis and treatment. Oncologist 12(Suppl):11–21
Dittner AJ, Wessely SC, Brown RG (2004) The assessment of fatigue. A practical guide for clinicans and researchers. J Psychosom Res 56:157–170
Cook C (2010) Mode of administration bias. J Man Manip Ther 18(2):61–63
Bowling A (2005) A mode of questionnaire administration can have serious effects on data quality. J Public Health (Oxf) 27(3):281–291
Mock V, Atkinson A, Barsevick A, Cella D, Cimprich B, Cleeland C et al (2000) NCCN practice guidelines for cancer related fatigue. Oncology (Williston Park) 14(11A):151–161
Minton O, Stone P (2009) A systematic review of the scales used for the management of cancer-related fatigue (CRF). Ann Oncol 20:17–25. doi:10.1093/annonc/mdn537
Whiting P, Rutjes A, Reitsma J B, Bossuyt PM, Kleijnen J (2003) The development of QUADAS: a tool for the quality of assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol, 3–25. doi: 10.1186/1471-2288-3-25
Manias E, Beanland CJ, Riley RG, Hutchinson A (2006) Development and validation of the self-administration of medication tool. Ann Pharmacother 40(6):1064–1073. doi:10.1345/aph.1G677
Grant JS, Kinney MR (1992) Using the Delphi technique to examine the content validity of nursing diagnoses. International Journal of Nursing Terminologies and Classfications 3(1):12–22. doi:10.1111/j.1744-618X.1992.tb00193.x
Vidhubala E, Latha S, Kannan RR, Mani CS, Karthikesh K, Muthuvel R et al (2005) Validation of quality of life questionnaire for patients with cancer—Indian scenario. Indian J Cancer 42(3):139–144. doi:10.4103/0019-509X.17058
Zigmond AS, Snaith RP (1983) The hospital anxiety and depression scale. Acta Psychiatr Scand 67(6):361–370
Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ (1988) The Pittsburgh sleep quality index: a new instrument for psychatric practice and research. Psychiatry Res 28:193–213. doi:10.1016/0165-1781(89)90047-4
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ et al (1993) The European Organization for Research and Treatment of cancer QLQ-C30: a quality of life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85(5):365–376. doi:10.1093/jnci/85.5.365
Nunnally J, Bernstein I (1994) Psychometric theory. McGraw-Hill, New York
Andresen EM (2000) Criteria for assessing the tools of disability outcomes research. Arch Phys Med Rehabil 81(2):S15–S20. doi:10.1053/apmr.2000.20619
Ferketich, S. (1991). Focus on Psychometrics: aspects of item analysis. Res Nursing Health, 165–68
Field AP (2005) Discovering statistics usig SPSS, 2nd edn. SAGE, London
Kaiser HF (1974) An index of factorial simplicity. Psychometrika 39:31–36
Ahlberg K, Ekman T, Gaston-Johansson F, Mock V (2003) Assessment and management of cancer-related fatigue in adults. Lancet 362(9384):640–650. doi:10.1016/S0140-6736(03)14186-4
Schwartz AL (2008) Daily fatigue patterns and effect of exercise in women in breast cancer. Cancer Pract 8(1):16–24
Jacobsen P (2004) Assessment of fatigue in cancer patients. J Natl Cancer Inst Monogr 32:93–97. doi:10.1093/jncimonographs/lgh010
Breitbart W S, Alici Y (2010) Fatigue. In J. C. Holland, W. S. Breitbart, P. B. Jacobsen, M. S. Lederberg, J. Loscalzo, & R. McCorkle, Psycho-oncology (p. 236). New York: Oxford University Press
Curt GA, Breitbart W, Cella D, Groopman JE, Horning SJ, Itri LM et al (2000) Impact of cancer-related fatigue on the lives of patients: new findings from the fatigue coalition. Oncologist 5(5):353–360. doi:10.1634/theoncologist.5-5-353
Roscoe JA, Kaufman ME, Matteson-Rusby SE, Palesh OG, Ryan JL, Kohli S et al (2007) Cancer-related fatigue and sleep disorders. Oncologist 12(suppl 1):35–42
Brown LF, Kroenke K (2009) Cancer-related fatigue and its associations with depression and anxiety: a systematic review. Psychosomatics 50(5):440–447
Stone P, Richard M, A’Hern R, Hardy J (2009) A study to investiagte the prevalence, severity and correlates of cancer-related fatigue (CRF). Ann Oncol, 17–25. doi: 10.1023/A:1008331230608
Loge JH, Abrahamsen AF, Ekeberg O, Kaasa S (1999) Hodgkin’s disease survivors more fatigued than the general population. J Clin Oncol 17(1):253–261
Acknowledgements
The authors are thankful to Dr. Latha Satish, Dr. R Swaminathan, Mr. C Sundaramoorthy, Ms. S Vijayalakshmi, Mr. D Prabhakar, and other members of the Department of Psycho-oncology at Cancer Institute (WIA) for their assistance during this study. In addition, we are grateful to all the experts and patients for their valuable contribution during the development of the tool.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
None.
Funding
None.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Jeyaram, S., Veeraiah, S. & Elangovan, V. Validation of the symbolic assessment of fatigue extent (SAFE)—a cancer fatigue tool with visual response formats. Support Care Cancer 25, 1111–1119 (2017). https://doi.org/10.1007/s00520-016-3499-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-016-3499-1