Abstract
Purpose
Anthracycline and ifosfamide-based chemotherapy represents a widely used regimen both in early and advanced settings in soft tissue sarcoma (STS). Prophylaxis with granulocyte colony-stimulating factor (G-CSF) reduces the severity of chemotherapy-induced neutropenia. The aim of this study was to assess the efficacy and safety of biosimilar G-CSF in these patients.
Methods
Between 2003 and 2013, 67 patients with soft tissue tumors under epirubicin and ifosfamide (EI) treatment receiving biosimilar filgrastim (Zarzio®), originator filgrastim (Granulokine®, Neupogen®), and lenograstim (only originator Myelostim®) as primary prophylaxis for a total of 260 cycles of therapy were retrospectively analyzed. Baseline patient characteristics were summarized in a propensity score (PS).
Results
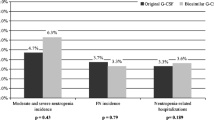
The incidence of febrile neutropenia (FN) was 44.0 % in biosimilar filgrastim, 40.0 % in originator filgrastim, and 45.5 % in the lenograstim groups (p = 0.935). All grade and G4 neutropenia were similar in the three groups with the same safety profile. The use of biosimilar filgrastim achieved cost savings of €225.25 over originator filgrastim and €262.00 over lenograstim.
Conclusion
Biosimilar G-CSF was effective in preventing FN and in reducing the need for hospitalization in STS patients undergoing EI treatment. It also proved comparable to its reference products from both a clinical and cost-effective standpoint.


Similar content being viewed by others
References
Enzinger FM, Weiss SW (1988) General considerations in soft tissue tumors. St Louis, MO, Mosby, pp. 1–18
Stiller CA, Trama A, Serraino D, et al (2012) Descriptive epidemiology of sarcomas in Europe: report from the RARECARE project. Eur J Cancer
Suit HD (1995) Tumors of the connective and supporting tissues. Radiother Oncol 34:93–104
Frustaci S, Gherlinzoni F, De Paoli A, et al. (2001) Adjuvant chemotherapy for adult soft tissue sarcomas of the extremities and girdles: results of the Italian randomized cooperative trial. J Clin Oncol 19:1238–1247
Edmonson JH, Ryan LM, Blum RG, et al. (1993) Randomized comparison of doxorubicin alone versus ifosfamide plus doxorubicin or mitomycin, doxorubicin and cisplatin against advanced soft tissue sarcoma. J Clin Oncol 7:1269–1275
Bui BN, Chevallier B, Chevreau C, et al. (1995) Efficacy of lenograstim on hematologic tolerance to MAID chemotherapy in patients with advanced soft tissue sarcoma and consequences on treatment dose intensity. J Clin Oncol 13:2629–2633
Woll PJ, Reichardt P, Le Cesne A, et al. (2012) Adjuvant chemotherapy with doxorubicin, ifosfamide, and lenograstim for resected soft-tissue sarcoma (EORTC 62931): a multicentre randomised controlled trial. Lancet Oncol 13:1045–1054
Aapro M, Bohlius J, Cameron D, Dal LL, et al. (2011) 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer 47:8–32
Sivgin S, Karakus E, Kaynar L, et al. (2013) The comparison of Filgrastim (Neupogen®), biosimilar filgrastim (Leucostim®) and Lenograstim (Granocyte®) as first line peripheral blood stem cell mobilization strategy in autologous hematopoieitic stem cell transplantation: a single center experience from Turkey. Transfus Apher Sci 48:315–320
Ianotto JC, Tempescul A, Yan X, et al. (2012) Experience (1 year) of G-CSF biosimilars in PBSCT for lymphoma and myeloma patients. Bone Marrow Transplant 47:874–876
Common Terminology criteria for adverse events (CTCAE) v.4.03. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_520_3390.docx 5x7.pdf Accessed August 24, 2012
McCaffrey DF, Griffin BA, Almirall D, et al. (2013) A tutorial on propensity score estimation for multiple treatments using generalized boosted models. Stat Med 32:3388–3414
Gronchi A, Frustaci S, Mercuri M, et al. (2012) Short, full-dose adjuvant chemotherapy in high-risk adult soft tissue sarcomas: a randomized clinical trial from the Italian sarcoma group and the Spanish sarcoma group. J Clin Oncol 10:850–856
European Medicines Agency. Annex to guideline on similar biological medicinal products containing biotechnology-derived proteins as active substance: nonclinical and clinical issues. Guidance on similar medicinal products containing recombinant granulocyte-colony stimulating factor [EMEA/CHMP/BMWP/31329/2005]. European Medicines Agency London, 2006. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003955.pdf Accessed August 24 2012
Sörgel F, Lerch H, Lauber T (2010) Physicochemical and biologic comparability of a biosimilar granulocyte colony-stimulating factor with its reference product. Bio Drugs 24:347–357
Gascon P, Tesh H, Verpoort K, et al. (2013) Clinical experience with Biosimilar Filgrastim® in Europe: what have we learned? Support Care Cancer 21:2925–2932
Acknowledgments
The authors thank Cristiano Verna for editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Funding
None.
Rights and permissions
About this article
Cite this article
Bongiovanni, A., Monti, M., Foca, F. et al. Recombinant granulocyte colony-stimulating factor (rG-CSF) in the management of neutropenia induced by anthracyclines and ifosfamide in patients with soft tissue sarcomas (NEUSAR). Support Care Cancer 25, 111–117 (2017). https://doi.org/10.1007/s00520-016-3390-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-016-3390-0