Abstract
Purpose
To evaluate the effectiveness and tolerability profile of tapentadol prolonged release (PR) in a cohort of head and neck cancer (HNC) patients affected by background pain due to painful mucositis during intensity modulated radiation therapy with or without cisplatin with definitive and adjuvant intent.
Materials and methods
Tapentadol PR was administered at the moment of pain onset in opioid-naive patients at the dosage of 50 mg BID. The dosage was increased 50 mg twice a day until the optimal dose of no more than 500 mg/day of tapentadol PR. Primary endpoint of the analysis was the evaluation of improved assessment using the numerical rating scale (NRS). Secondary endpoints were as follows: (1) assessment of the treatment received using the patients’ global impression of change (PGIC) scale; (2) weight increase/stability; (3) sleep quality; and (4) tolerability. The period of observation was 90 days from the start of antineoplastic treatment.
Results
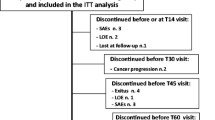
Between September 2014 and May 2015, 30 HNC patients were observed. The average age was 64.9 years (range, 36–80). Twenty-two days after the start of antineoplastic treatment, tapentadol PR was administered to 25 % of patients. This percentage was increased to 50 % after 39 days and to 75 % after 43 days. Considering the efficacy of tapentadol PR on daily pain, there was a reduction of 30 % (95 % C.I. 69.3 ÷ 96.2 %) in the pain score in 26 patients (86.7 %), and a reduction of 50 % (95 % C.I. 57.7 ÷ 90.1 %) in 23 patients (76.7 %).
Conclusion
The use of tapentadol PR is feasible and well tolerated in HNC patients affected by background pain due to painful mucositis during intensity modulated radiotherapy with or without cisplatin. Further studies are needed to enhance current findings.

Similar content being viewed by others
References
Elting LS, Keefe DM, Sonis ST et al (2008) Patient-reported measurements of oral mucositis in head and neck cancer patients treated with radiotherapy with or without chemotherapy: demonstration of increased frequency, severity, resistance to palliation, and impact on quality of life. Cancer 113:2704–2713
Bossi P, Locati L, Bergamini C, Mirabile A, Granata R, Imbimbo M et al (2014) Fentanyl pectin nasal spray as treatment for incident predictable breakthrough pain (BTP) in oral mucositis induced by chemoradiotherapy in head and neck cancer. Oral Oncol 50(9):884–887
Epstein JB, Wilkie DJ, Fischer DJ, Kim YO, Villines D (2009) Neuropathic and nociceptive pain in head and neck cancer patients receiving radiation therapy. Head Neck Oncol 1:26
Benoleil R, Epstein J, Eliav E, Jurevic R, Elad S (2007) Orofacial pain in cancer: part I: mechanisms. J Dent Res 86(6):491–505
Kress HG, Koch ED, Kosturski H et al (2014) Tapentadol prolonged release for managing moderate to severe, chronic malignant tumor-related pain. Pain Physician 17:329–343
Schroder W, Tzschentke TM, Terlinden R et al (2011) Synergistic interaction between the two mechanisms of action of tapentadol in analgesia. J Pharmacol Exp Ther 337:312–320
Tzschentke TM, Christoph T, Kogel BY (2014) The muopioid receptor agonist/noradrenaline reuptake inhibition (MOR-NRI) concept in analgesia: the case of tapentadol. CNS Drugs 28:319–329
Grégoire V, Langendijk JA, Nuyts S (2015) Advances in radiotherapy for head and neck cancer. J Clin Oncol 33(29):3277–3284
Mazzola R, Ferrera G, Alongi F, Mannino M, Abbate B, Cucchiara T, Iacoviello G, Sciumè F, Di Paola G, Federico M, Blasi L, Lo Casto A, Lagalla R, Messana D (2015) Organ sparing and clinical outcome with step-and-shoot IMRT for head and neck cancer: a mono-institutional experience. Radiol Med 120(8):753–758
Mazzola R, Ricchetti F, Fiorentino A, Fersino S, Giaj Levra N, Naccarato S, Sicignano G, Albanese S, Di Paola G, Alterio D, Ruggieri R, Alongi F (2014) Dose-volume-related dysphagia after constrictor muscles definition in head and neck cancer intensity-modulated radiation treatment. Br J Radiol 87(1044):20140543
Christianen ME, Langendijk JA, Westerlaan HE, van de Water TA, Bijl HP (2011) Delineation of organs at risk involved in swallowing for radiotherapy treatment planning. Radiother Oncol 101(3):394–402
Trotti A, Bellm LA, Epstein JB et al (2003) Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: a systematic literature review. Radiother Oncol 66:253–262
Mazzola R, Ricchetti F, Fersino S, Fiorentino A, Giaj Levra N, Di Paola G, Ruggieri R, Alongi F (2016) Predictors of mucositis in oropharyngeal and oral cavity cancer in patients treated with volumetric modulated radiation treatment: a dose-volume analysis. Head Neck 38(Suppl 1):E815–E819
Benyamin R, Trescot AM, Datta S et al (2008) Opioid complications and side effects. Pain Physician 11:S105–S120
Bossi P, Numico G, De Santis V, Ruo Redda MG, Reali A, Belgioia L et al (2014) Prevention and treatment of oral mucositis in patients with head and neck cancer treated with (chemo) radiation: report of an Italian survey. Support Care Cancer 22(7):1889–1896
Tzschentke TM, Jahnel U, Kogel B et al (2009) Tapentadol hydrochloride: a next-generation, centrally acting analgesic with two mechanisms of action in a single molecule. Drugs Today (Barc) 45:483–496
Baron R, Martin-Mola E, Müller M, Dubois C, Falke D, Steigerwald I (2015) Effectiveness and safety of tapentadol prolonged release (PR) versus a combination of tapentadol PR and pregabalin for the management of severe, chronic low back pain with a neuropathic component: a randomized, double-blind, phase 3b study. Pain Pract 15(5):455–470
González Ferreira JA, Jaén Olasolo J, Azinovic I, Jeremic B (2015) Effect of radiotherapy delay in overall treatment time on local control and survival in head and neck cancer: review of the literature. Rep Pract Oncol Radiother 20(5):328–339
Riemsma R, Forbes C, Harker J, Worthy G, Misso K, Schäfer M, Kleijnen J, Stürzebecher S (2011) Systematic review of tapentadol in chronic severe pain. Curr Med Res Opin 27(10):1907–1930, Erratum in: Curr Med Res Opin 2012; 28(1):148
Buglione M, Cavagnini R, Di Rosario F, Maddalo M, Vassalli L, Grisanti S et al (2016) Oral toxicity management in head and neck cancer patients treated with chemotherapy and radiation: xerostomia and trismus (part 2). Literature review and consensus statement. Crit Rev Oncol Hematol 102:47–54
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Rosario, M., Francesco, R., Sergio, F. et al. Effectiveness of tapentadol prolonged release for the management of painful mucositis in head and neck cancers during intensity modulated radiation therapy. Support Care Cancer 24, 4451–4455 (2016). https://doi.org/10.1007/s00520-016-3351-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-016-3351-7