Abstract
Purpose
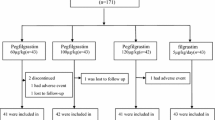
To evaluate the safest timing of pegfilgrastim administration in dose-dense anthracycline- and taxane-based chemotherapy, three different cohorts of patients enrolled in the Gruppo Italiano Mammella (GIM) 2 study and treated at the coordinating center received pegfilgrastim 24 h (cohort A) or 72 h (cohort B) or 96 h (cohort C) after chemotherapy.
Methods
A total of 41 patients were included. The safety of pegfilgrastim administration in terms of occurrence of early and late leukocytosis and the behavior of white blood cells (WBC) counts in the three cohorts across all chemotherapy cycles were evaluated. Anthracycline and taxane cycles were analyzed separately.
Results
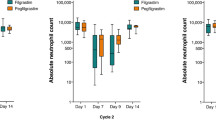
The occurrence of early leukocytosis was a more common event in patients in cohort A in both anthracycline and taxane cycles (75 and 66.7 %) as compared to cohort B (50 and 60 %) and cohort C (66.7 and 33.3 %). More patients in cohort C developed late leukocytosis in both anthracycline and taxane cycles (50 and 100 %) as compared to cohort A (0 and 66.7 %) and cohort B (35.7 and 86.7 %). Patients in cohort A experienced the highest median value of WBC count 24 h after pegfilgrastim administration in both anthracycline and taxane cycles (61.2 × 103/μl and 67.8 × 103/μl). Patients in cohort C experienced the highest median value of WBC count at day 13 in both anthracycline and taxane cycles (19.4 × 103/μl and 24.2 × 103/μl).
Conclusions
For the prevention of leukocytosis, the safest timing of pegfilgrastim administration based on WBC count in dose-dense anthracycline- and taxane-based regimens seems to be 72 h after chemotherapy.
Trial registration
This study is registered with https://clinicaltrials.gov/ct2/show/NCT00433420.


Similar content being viewed by others
References
Bonilla L, Ben-Aharon I, Vidal L, et al (2010) Dose-dense chemotherapy in nonmetastatic breast cancer: a systematic review and meta-analysis of randomized controlled trials. J Natl Cancer Inst 102:1845–1854
Aapro MS, Bohlius J, Cameron DA, et al (2011) 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer 47:8–32
Crawford J, Caserta C, Roila F, Guidelines Working Group ESMO (2010) Hematopoietic growth factors: ESMO Clinical Practice Guidelines for the applications. Ann Oncol 21(Suppl 5):v248–v251
Smith TJ, Khatcheressian J, Lyman GH, et al (2006) 2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol 24:3187–3205
Yang B-B, Kido A (2011) Pharmacokinetics and pharmacodynamics of pegfilgrastim. Clin Pharmacokinet 50:295–306
Holmes FA, O’Shaughnessy JA, Vukelja S, et al (2002) Blinded, randomized, multicenter study to evaluate single administration pegfilgrastim once per cycle versus daily filgrastim as an adjunct to chemotherapy in patients with high-risk stage II or stage III/IV breast cancer. J Clin Oncol 20:727–731
Green MD, Koelbl H, Baselga J, et al (2003) A randomized double-blind multicenter phase III study of fixed-dose single-administration pegfilgrastim versus daily filgrastim in patients receiving myelosuppressive chemotherapy. Ann Oncol 14:29–35
Siena S, Piccart MJ, Holmes FA, et al (2003) A combined analysis of two pivotal randomized trials of a single dose of pegfilgrastim per chemotherapy cycle and daily filgrastim in patients with stage II-IV breast cancer. Oncol Rep 10:715–724
Burstein HJ, Parker LM, Keshaviah A, et al (2005) Efficacy of pegfilgrastim and darbepoetin alfa as hematopoietic support for dose-dense every-2-week adjuvant breast cancer chemotherapy. J Clin Oncol 23:8340–8347
Arshad M, Seiter K, Bilaniuk J, et al (2005) Side effects related to cancer treatment: CASE 2. Splenic rupture following pegfilgrastim. J Clin Oncol 23:8533–8534
Watring NJ, Wagner TW, Stark JJ (2007) Spontaneous splenic rupture secondary to pegfilgrastim to prevent neutropenia in a patient with non-small-cell lung carcinoma. Am J Emerg Med 25:247–248
Hershman D, Neugut AI, Jacobson JS, et al (2007) Acute myeloid leukemia or myelodysplastic syndrome following use of granulocyte colony-stimulating factors during breast cancer adjuvant chemotherapy. J Natl Cancer Inst 99:196–205
Del Mastro L, De Placido S, Bruzzi P, et al (2015) Fluorouracil and dose-dense chemotherapy in adjuvant treatment of patients with early-stage breast cancer: an open-label, 2 × 2 factorial, randomised phase 3 trial. Lancet 385:1863–1872
Giles FJ, Shen Y, Kantarjian HM, et al (2001) Leukapheresis reduces early mortality in patients with acute myeloid leukemia with high white cell counts but does not improve long- term survival. Leuk Lymphoma 42:67–73
Ganzel C, Becker J, Mintz PD, et al (2012) Hyperleukocytosis, leukostasis and leukapheresis: practice management. Blood Rev 26:117–122
Senkus E, Kyriakides S, Penault-Llorca F et al (2013) Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 24 Suppl 6:vi7–23.
NCCN Clinical Practice Guidelines in Oncology. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#breast. Accessed 20 July 2015.
Rossi L, Tomao F, Lo Russo G, et al (2013) Efficacy and safety analysis of once per cycle pegfilgrastim and daily lenograstim in patients with breast cancer receiving adjuvant myelosuppressive chemotherapy FEC 100: a pilot study. Ther Clin Risk Manag 9:457–462
Lambertini M, Del Mastro L, Bellodi A, Pronzato P (2014) The five “Ws” for bone pain due to the administration of granulocyte-colony stimulating factors (G-CSFs). Crit Rev Oncol Hematol 89:112–128
Engert A, Bredenfeld H, Döhner H, et al (2006) Pegfilgrastim support for full delivery of BEACOPP-14 chemotherapy for patients with high-risk Hodgkin’s lymphoma: results of a phase II study. Haematologica 91:546–549
Zwick C, Hartmann F, Zeynalova S, et al (2011) Randomized comparison of pegfilgrastim day 4 versus day 2 for the prevention of chemotherapy-induced leukocytopenia. Ann Oncol 22:1872–1877
Whitworth JM, Matthews KS, Shipman KA, et al (2009) The safety and efficacy of day 1 versus day 2 administration of pegfilgrastim in patients receiving myelosuppressive chemotherapy for gynecologic malignancies. Gynecol Oncol 112:601–604
Burris HA, Belani CP, Kaufman PA, et al (2010) Pegfilgrastim on the same day versus next day of chemotherapy in patients with breast cancer, non-small-cell lung cancer, ovarian cancer, and non-Hodgkin’s lymphoma: results of four multicenter, double-blind, randomized phase II studies. J Oncol Pract 6:133–140
Loibl S, Mueller V, von Minckwitz G, et al (2011) Comparison of pegfilgrastim on day 2 vs. day 4 as primary prophylaxis of intense dose-dense chemotherapy in patients with node-positive primary breast cancer within the prospective, multi-center GAIN study: (GBG 33). Support Care Cancer 19:1789–1795
Skarlos DV, Timotheadou E, Galani E, et al (2009) Pegfilgrastim administered on the same day with dose-dense adjuvant chemotherapy for breast cancer is associated with a higher incidence of febrile neutropenia as compared to conventional growth factor support: matched case-control study of the Hellenic Cooperative Oncology Group. Oncology 77:107–112
Acknowledgments
We thank all the study participants as well as all the study personnel and investigators. This paper is dedicated to the memory of Dr. Marco Venturini, leader of the Gruppo Italiano Mammella (GIM) group, who left us too early.
Conflict of interest
ML received a 2014 Conquer Cancer Foundation of ASCO Merit Award for the presentation at the 2014 ASCO Annual Meeting. PP disclosed advisory role for Amgen Dompè SPA. All the other authors have no conflict of interest to disclose. The main GIM2 study received funding by Bristol-Meyers Squibb, Pharmacia, and Dompè Biotec, Italy. This ancillary study did not receive additional funding.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lambertini, M., Bruzzi, P., Poggio, F. et al. Pegfilgrastim administration after 24 or 72 or 96 h to allow dose-dense anthracycline- and taxane-based chemotherapy in breast cancer patients: a single-center experience within the GIM2 randomized phase III trial. Support Care Cancer 24, 1285–1294 (2016). https://doi.org/10.1007/s00520-015-2907-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-015-2907-2