Abstract
Purpose
The aim of this study was to compare the effectiveness of prophylactic single fixed dose of pegfilgrastim and daily administration of filgrastim on febrile neutropenia (FN), severe neutropenia, treatment delay, and dose reduction in patients with breast cancer receiving dose-dense adjuvant chemotherapy.
Methods
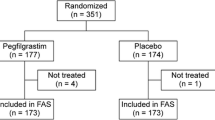
A retrospective cohort study with 1058 breast cancer patients matched by age and chemotherapy was conducted. The primary endpoints were FN, severe (grade 3, 4) neutropenia, dose reduction (>10 % reduction of the dose planned), and treatment delay (dose given more than 2 days later).
Results
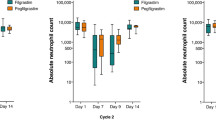
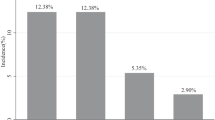
Eighteen episodes of FN (3.4 %) in the filgrastim group and 23 (4.3 %) in the pegfilgrastim group (p = 0.500) were recorded. More than half of the total episodes (27/41) occurred during the first 4 cycles of treatment. Patients who received filgrastim were almost three times more likely to experience a severe neutropenia episode and were significantly more likely to experience a dose reduction (18.5 %) compared to those who received pegfilgrastim (10.8 %) (p < 0.001). The percentage of patients, who received their planned dose on time, was significantly lower in patients receiving filgrastim (58 %) compared to those receiving pegfilgrastim (72.4 %, p < 0.001).
Conclusions
No significant difference was detected on FN rate between daily administration of filgrastim and single administration of pegfilgrastim. However, patients receiving pegfilgrastim had a significantly lower rate of severe neutropenia, as well as dose reduction and treatment delay, thus, achieving a higher dose density.
Similar content being viewed by others
References
Lyman GH, Lyman CH, Agboola O (2005) Risk models for predicting chemotherapy-induced neutropenia. Oncologist 10:427–437
Crawford J (2006) Risk assessment and guidelines for first-cycle colony-stimulating factor use in the management of chemotherapy-induced neutropenia. Oncology 20:22–28
Kuderer NM, Dale DC, Crawford J, Cosler LE, Lyman GH (2006) Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer 106:2258–2266
Padilla G, Ropka ME (2005) Quality of life and chemotherapy-induced neutropenia. Cancer Nurs 28:167–171
Fortner BV, Schwartzberg L, Tauer K, Houts AC, Hackett J, Stolshek BS (2005) Impact of chemotherapy-induced neutropenia on quality of life: a prospective pilot investigation. Support Care Cancer 13:522–528
Chirivella I, Bermejo B, Insa A et al (2009) Optimal delivery of anthracycline-based chemotherapy in the adjuvant setting improves outcome of breast cancer patients. Breast Cancer Res Treat 114:479–484
Liou SY, Stephens JM, Carpiuc KT, Feng W, Botteman MF, Hay JW (2007) Economic burden of haematological adverse effects in cancer patients: a systematic review. Clin Drug Investig 27:381–396
Lyman GH (2005) Guidelines of the national comprehensive cancer network on the use of myeloid growth factors with cancer chemotherapy: a review of the evidence. J Natl Compr Canc Netw 3:557–571
Timmer-Bonte JN, Tjan-Heijnen VC (2006) Febrile neutropenia: highlighting the role of prophylactic antibiotics and granulocyte colony-stimulating factor during standard dose chemotherapy for solid tumors. Anticancer Drugs 17:881–889
Dale DC (2003) Optimizing the management of chemotherapy-induced neutropenia. Clin Adv Hematol Oncol 1:679–684
Aapro MS, Cameron DA, Pettengell R et al (2006) EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphomas and solid tumours. Eur J Cancer 42:2433–2453
Smith TJ, Khatcheressian J, Lyman GH et al (2006) 2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol 24:3187–3205
Kuderer NM, Dale DC, Crawford J, Lyman GH (2007) Impact of primary prophylaxis with granulocyte colony-stimulating factor on febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: a systematic review. J Clin Oncol 25:3158–3167
von Minckwitz G, Schwenkglenks M, Skacel T et al (2009) Febrile neutropenia and related complications in breast cancer patients receiving pegfilgrastim primary prophylaxis versus current practice neutropaenia management: results from an integrated analysis. Eur J Cancer 45:608–617
Pinto L, Liu Z, Doan Q, Bernal M, Dubois R, Lyman G (2007) Comparison of pegfilgrastim with filgrastim on febrile neutropenia, grade IV neutropenia and bone pain: a meta-analysis of randomized controlled trials. Curr Med Res Opin 23:2283–2295
Fountzilas G, Dafni U, Gogas H et al (2008) Postoperative dose-dense sequential chemotherapy with epirubicin, paclitaxel and CMF in patients with high-risk breast cancer: safety analysis of the Hellenic Cooperative Oncology Group randomized phase III trial HE 10/00. Ann Oncol 19:853–860
Fountzilas G, Pectasides D, Christodoulou C et al (2006) Adjuvant dose-dense sequential chemotherapy with epirubicin, CMF, and weekly docetaxel is feasible and safe in patients with operable breast cancer. Med Oncol 23:479–488
Skarlos DV, Timotheadou E, Galani E et al (2009) Pegfilgrastim administered on the same day with dose-dense adjuvant chemotherapy for breast cancer is associated with a higher incidence of febrile neutropenia as compared to conventional growth factor support: matched case-control study of the Hellenic Cooperative Oncology Group. Oncology 77:107–112
Cooper KL, Madan J, Whyte S, Stevenson MD, Akehurst RL (2011) Granulocyte colony-stimulating factors for febrile neutropenia prophylaxis following chemotherapy: systematic review and meta-analysis. BMC Cancer 11:404
Holmes FA, Jones SE, O’Shaughnessy J et al (2002) Comparable efficacy and safety profiles of once-per-cycle pegfilgrastim and daily injection filgrastim in chemotherapy-induced neutropenia: a multicenter dose-finding study in women with breast cancer. Ann Oncol 13:903–909
Green MD, Koelbl H, Baselga J et al (2003) A randomized double-blind multicenter phase III study of fixed-dose single-administration pegfilgrastim versus daily filgrastim in patients receiving myelosuppressive chemotherapy. Ann Oncol 14:29–35
Danova M, Chiroli S, Rosti G, Doan QV (2009) Cost-effectiveness of pegfilgrastim versus 6 days of filgrastim for preventing febrile neutropenia in breast cancer patients. Tumori 95:219–226
Lyman GH, Lalla A, Barron RL, Dubois RW (2009) Cost-effectiveness of pegfilgrastim versus filgrastim primary prophylaxis in women with early-stage breast cancer receiving chemotherapy in the United States. Clin Ther 31:1092–1104
Liu Z, Doan QV, Malin J, Leonard R (2009) The economic value of primary prophylaxis using pegfilgrastim compared with filgrastim in patients with breast cancer in the UK. Appl Health Econ Health Policy 7:193–205
Acknowledgments
The authors wish to thank all Hellenic Cooperative Oncology Group coordinators for data collection, Ms. E. Fragou for study monitoring, Ms. M. Moschoni for data management, and Mw. N. Sotiriadou for secretarial assistance.
Conflict of interest
There is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kourlaba, G., Dimopoulos, M.A., Pectasides, D. et al. Comparison of filgrastim and pegfilgrastim to prevent neutropenia and maintain dose intensity of adjuvant chemotherapy in patients with breast cancer. Support Care Cancer 23, 2045–2051 (2015). https://doi.org/10.1007/s00520-014-2555-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-014-2555-y