Abstract
Purpose
The purpose of this study is to determine the incidence of febrile neutropenia (FN) among women receiving FEC-D (flurouracil 500 mg/m2, epirubicin 100 mg/m2, and cyclophosphamide 500 mg/m2 every 3 weeks for three cycles followed by docetaxel 100 mg/m2 every 3 weeks for three cycles) chemotherapy for early stage breast cancer (ESBC) and the impact of primary granulocyte colony-stimulating factor (G-CSF) prophylaxis in a non-clinical trial setting.
Patients and methods
A retrospective chart review of women referred for ESBC to The Moncton Hospital between 2005 and 2010 evaluated patient and disease characteristics, adjuvant chemotherapy receipt, G-CSF usage, FN incidence, hospital admission rates, and length of stay. Association of variables with FN was examined, and exploratory multivariable logistic regression modeling examined the impact of baseline variables on risk of FN.
Results
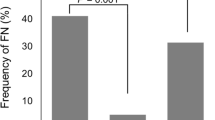
Of 520 patients enrolled in the database, 251 (48.3 %) received adjuvant chemotherapy for ESBC. Most (66.9 %) received FEC-D. Overall, 55 (21.9 %) patients developed FN. Forty-four (26.2 %) patients on FEC-D developed FN. Forty of 129 (31.0 %) FEC-D patients who did not receive primary G-CSF prophylaxis developed FN, versus 4 of 39 (10.3 %) receiving G-CSF. Receipt of FEC-D or TC (docetaxel 75 mg/m2 and cyclophosphamide 600 mg/m2 every 3 weeks for four or six cycles) was associated with odds ratios of 6.5 or 6.77, respectively, for the development of FN. Receipt of trastuzumab with chemotherapy was associated with an odds ratio of 3.48 for developing FN versus no trastuzumab. Primary G-CSF prophylaxis led to a 63 % reduction in the odds ratio of developing FN.
Conclusions
Incidence of FN with FEC-D treatment is considerably higher in clinical practice than reported in phase III trials. Consistent with ASCO guidelines, prophylactic G-CSF should be considered for all ESBC patients receiving adjuvant FEC-D.


Similar content being viewed by others
References
Canadian Cancer Society www.cancerca/canada-wide/about%20cancer/cancer%20statistics/stats%20at%20a%20glance/breast%20canceraspx. Accessed 4-Dec-2012
Bonadonna G, Valagussa P, Moliterni A, Zambetti M, Brambilla C (1995) Adjuvant cyclophosphamide, methotrexate, and fluorouracil in node-positive breast cancer: the results of 20 years of follow-up. N Engl J Med 332:901–906
Lyman GH, Rolston KVI (2010) How we treat febrile neutropenia in patients receiving cancer chemotherapy. J Oncol Pract 6(3):149–152
Smith TJ, Khatcheressian J, Lyman GH, Ozer H, Armitage JO, Balducci L, Bennett CL, Cantor SB, Crawford J, Cross SJ, Demetri G, Desch CE, Pizzo PA, Schiffer CA, Schwartzberg L, Somerfield MR, Somlo G, Wade JC, Wade JL, Winn RJ, Wozniak AJ, Wolff AC (2006) 2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol 24(19):3187–3205
Aapro MS, Bohlius J, Cameron DA, Dal Lago L, Donnelly JP, Kearney N, Lyman GH, Pettengell R, Tjan-Heijnen VC, Walewski J, Weber DC, Zielinski C, European Organisation for Research and Treatment of Cancer (2011) 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer 47(1):8–32
Roche H, Fumoleau P, Spielmann M, Canon JL, Delozier T, Serin D, Symann M, Kerbrat P, Soulie P, Eichler F, Viens P, Monnier A, Vindevoghel A, Campone M, Goudier MJ, Bonneterre J, Ferrero JM, Martin AL, Geneve J, Asselain B (2006) Sequential adjuvant epirubicin-based and docetaxel chemotherapy for node-positive breast cancer patients: the FNCLCC PACS 01 trial. J Clin Oncol 24(36):5664–5671
Rayson D, Lutes S, Sellon M, Colwell B, Dorreen M, Drucker A, Jeyakumar A, Snow S, Younis T (2012) Incidence of febrile neutropenia during adjuvant chemotherapy for breast cancer: a prospective study. Curr Oncol 19(3):e216–e218
Younis T, Rayson D, Thompson K (2012) Primary G-CSF prophylaxis for adjuvant TC or FEC-D chemotherapy outside of clinical trial settings: a systematic review and meta-analysis. Support Care Cancer 20(10):2523–2530
Hosmer DW, Lemeshow S (2000) Applied logistic regression. Wiley, New York
Akaike H (1974) A new look at the statistical model identification. IEEE Trans Autom Control 19(6):716–723
Symonds MRE, Moussalli A (2010) A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike’s information criterion. Behav Ecol Sociobiol 65:13–21
Raza S, Welch S, Younus J (2009) Relative dose intensity delivered to patients with early breast cancer: Canadian experience. Curr Oncol 16(6):8–12
Madarnas Y, Dent SF, Husain SF, Robinson A, Alkhayyat S, Hopman WM, Verreault JL, Vandenberg T (2011) Real-world experience with adjuvant FEC-D chemotherapy in four Ontario regional cancer centres. Curr Oncol 18(3):119–125
Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, Mackey J, Glaspy J, Chan A, Pawlicki M, Pinter T, Valero V, Liu MC, Sauter G, von Minckwitz G, Visco F, Bee V, Buyse M, Bendahmane B, Tabah-Fisch I, Lindsay MA, Riva A, Crown J (2011) Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med 365(14):1273–1283
Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE Jr, Davidson NE et al (2005) Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 353(16):1673–1684
Herceptin Product Monograph. Mississauga: Hoffmann-La Roche Ltd, 2013 Contract No.: Submission Control No 166449
Valachis A, Davide M, Nikolaos NP, Chlouverakis G, Mavroudis D, Georgoulias V (2011) Trastuzumab combined to neoadjuvant chemotherapy in patients with HER2-positive breast cancer: a systematic review and meta-analysis. Breast 20:485–490
Andersson M, Lidbrink E, Bjerre K, Wist E, Enevoldsen K, Jensen AB, Karlsson P, Tange UB, Sorensen PG, Moller S, Bergh J, Langkjer ST (2011) Phase III randomized study comparing docetaxel plus trastuzumab with vinorelbine plus trastuzumab as first-line therapy of metastatic or locally advanced human epidermal growth factor receptor 2-positive breast cancer: the HERNATA study. J Clin Oncol 29(3):264–271
Viani GA, Afonso SL, Stefano EJ, De Fendi LI, Soares FV (2007) Adjuvant trastuzumab in the treatment of HER-2-positive early breast cancer: a meta-analysis of published randomized trials. BMC Cancer 7:153–164
Harris CA, Ward RL, Dobbins TA, Drew AK, Pearson S (2011) The efficacy of HER-2 targeted agents in metastatic breast cancer: a meta-analysis. Ann Oncol 22(6):1308–1317
Acknowledgments
The authors gratefully acknowledge Tracey Allen, RN, for her assistance in patient identification and Dominique Richard for the expert administrative assistance. The authors also thank Dagmar Gross for the assistance with preparation of this manuscript.
Conflict of interest
Dr. H. Assi is a consultant for Amgen Canada Inc. and received research funding from Amgen Canada Inc. The remaining authors have no conflicts of interest to declare. As a courtesy, the manuscript was sent to Amgen Canada Inc. for review prior to submission, but no modifications were made based on their review. The opinions and analysis presented here are solely those of the authors. The authors have full control of all primary data and agree to allow the journal to review the data, if requested.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was conducted at The Moncton Hospital, Moncton, New Brunswick, Canada.
Rights and permissions
About this article
Cite this article
Assi, H., Murray, J., Boyle, L. et al. Incidence of febrile neutropenia in early stage breast cancer patients receiving adjuvant FEC-D treatment. Support Care Cancer 22, 3227–3234 (2014). https://doi.org/10.1007/s00520-014-2318-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-014-2318-9