Abstract
Purpose
Prevalence data of herpes simplex virus (HSV) in oral mucositis in children on treatment for cancer is limited. Quantitative polymerase chain reaction (PCR) has been seldom utilized for detection of HSV-1/2 in oral mucosa.
Methods
Children on treatment for cancer with oral mucositis were enrolled as cases and healthy children as controls. An oral swab from the lesion in cases and mucosal scraping in controls were obtained. Both qualitative and real-time quantitative PCR for HSV-1/2 were performed. Serum ELISA-IgG/IgM for HSV-1/2 antibodies (NovaLisa™-Dietzenbach-Germany) were measured.
Results
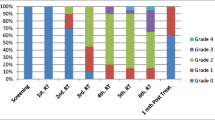
Thirty-two cases (Age, 6.3 ± 3.4 years) and 30 controls were enrolled. Majority (69 %) of cases had ALL. All patients had febrile neutropenia, except two. ELISA-IgM-HSV-1/2 was not positive in any case or control. ELISA-IgG-HSV-1/2 was positive in 11 (34 %) cases and nine (30 %) controls (p = 1.0). Qualitative PCR for HSV-1 detected the virus in eight (25 %) cases and nil controls (p = 0.009). HSV-2 was not detected in any case/control by qualitative PCR. Quantitative PCR detected HSV-1 in 21 (66 %) and HSV-2 in 22 (69 %) cases. In controls, quantitative PCR detected HSV-1 in three (10 %) and HSV-2 in none. In patients, the mean viral load of HSV-1 (5,500 ± 15,987 × 104 copies/nanogram DNA) was more than HSV-2 (4.03 ± 8.5 × 104) (p = 0.11). There was no correlation of HSV-1/2 with grading of mucositis.
Conclusions
Both HSV-1/2 are commonly shed from oral mucosal lesions in children receiving chemotherapy. In a novel finding, real-time PCR detected copies of HSV-2 in 69 % cases, all missed by conventional PCR. Implication for morbidity, if any, or treatment needs to be determined.
Similar content being viewed by others
References
Fulton J, Middleton G, McPhail J (2002) Management of oral complications. Semin Oncol Nurs 18:28–35
Dodd M, Miaskowski C, Dibble S et al (2000) Factors influencing oral mucositis in patients receiving chemotherapy. Cancer Pract 8:291–297
Elad S, Zadik Y, Hewson I et al (2010) Viral Infections Section, Oral Care Study Group, Multinational Association of Supportive Care in Cancer (MASCC)/International Society of Oral Oncology (ISOO). A systematic review of viral infections associated with oral involvement in cancer patients: a spotlight on Herpesviridea. Support Care Cancer 18:993–1006
Glenny AM, Gibson F, Auld E et al (2010) Children’s Cancer and Leukaemia Group (CCLG)/Paediatric Oncology Nurses Forum’s (CCLG-PONF) Mouth Care Group. The development of evidence-based guidelines on mouth care for children, teenagers and young adults treated for cancer. Eur J Cancer 46:1399–1412
Djuric M, Jankovic L, Jovanovic T et al (2009) Prevalence of oral herpes simplex virus reactivation in cancer patients: a comparison of different techniques of viral detection. J Oral Pathol Med 38:167–173
Stanberry LR (2011) Herpes simplex virus. In: Kliegman RM, Stanton BF, StGeme JW, Schor NF, Behrman RE (eds) Nelson textbook of pediatrics, 19th edn. Saunders, Philadelphia, pp 1097–1104
Usatine RP, Tinitigan R (2010) Nongenital herpes simplex virus. Am Fam Physician 82:1075–1082
Sandoval RL, Koga DH, Buloto LS, Suzuki R, Dib LL (2003) Management of chemo- and radiotherapy induced oral mucositis with low-energy laser: initial results of A.C. Camargo Hospital. J Appl Oral Sci 11:337–341
Figliolia SL, Oliveira DT, Pereira MC et al (2008) Oral mucositis in acute lymphoblastic leukaemia: analysis of 169 paediatric patients. Oral Dis 14:761–766
Childers NK, Stinnett EA, Wheeler P et al (1993) Oral complications in children with cancer. Oral Surg Oral Med Oral Pathol 75:41–47
Dreizen S, McCredie KB, Bodey GP, Keating MJ (1986) Quantitative analysis of the oral complications of antileukemia chemotherapy. Oral Surg Oral Med Oral Pathol 62:650–653
Greenberg MS (1996) Herpesvirus infections. Dent Clin North Am 40:359–368
Heimdahl A (1999) Prevention and management of oral infections in cancer patients. Support Care Cancer 7:224–228
Baglin TP, Gray JJ, Marcus RE, Wreghitt TG (1989) Antibiotic resistant fever associated with herpes simplex virus infection in neutropenic patients with haematological malignancy. J Clin Pathol 42:1255–1258
Lönnqvist B, Palmblad J, Ljungman P et al (1993) Oral acyclovir as prophylaxis for bacterial infections during induction therapy for acute leukaemia in adults. The Leukemia Group of Middle Sweden. Support Care Cancer 1:139–144
Hann IM, Prentice HG, Blacklock HA et al (1983) Acyclovir prophylaxis against herpes virus infections in severely immunocompromised patients: randomised double blind trial. Br Med J (Clin Res Ed) 287:384–388
Rubenstein EB, Peterson DE, Schubert M et al (2004) Mucositis study section of the multinational association for supportive care in cancer; international society for oral oncology. Clinical practice guidelines for the prevention and treatment of cancer therapy-induced oral and gastrointestinal mucositis. Cancer 100(9 Suppl):2026–2046
de Mendonça RM, de Araújo M, Levy CE et al (2012) Prospective evaluation of HSV, Candida spp., and oral bacteria on the severity of oral mucositis in pediatric acute lymphoblastic leukemia. Support Care Cancer 20:1101–1107
Ramphal R, Grant RM, Dzolganovski B et al (2007) Herpes simplex virus in febrile neutropenic children undergoing chemotherapy for cancer: a prospective cohort study. Pediatr Infect Dis J 26:700–704
Sepúlveda E, Brethauer U, Rojas J, Fernández E, Le Fort P (2005) Oral ulcers in children under chemotherapy: clinical characteristics and their relation with herpes simplex virus type 1 and Candida albicans. Med Oral Patol Oral Circ Bucal 10(Suppl 1):E1–E8
Anirudhan D, Bakhshi S, Xess I, Broor S, Arya LS (2008) Etiology and outcome of oral mucosal lesions in children on chemotherapy for acute lymphoblastic leukemia. Indian Pediatr 45:47–51
Carrega G, Castagnola E, Canessa A et al (1994) Herpes simplex virus and oral mucositis in children with cancer. Support Care Cancer 2:266–269
Chayavichitsilp P, Buckwalter JV, Krakowski AC, Friedlander SF (2009) Herpes simplex. Pediatr Rev 30:119–129
Lafferty WE, Coombs RW, Benedetti J, Critchlow C, Corey L (1987) Recurrences after oral and genital herpes simplex virus infection. Influence of site of infection and viral type. N Engl J Med 316:1444–1449
Wald A, Huang ML, Carrell D, Selke S, Corey L (2003) Polymerase chain reaction for detection of herpes simplex virus (HSV) DNA on mucosal surfaces: comparison with HSV isolation in cell culture. J Infect Dis 188:1345–1351
Augenbraun M, Corey L, Reichelderfer P et al (2001) Women’s Health Studies 002 Study Group. Herpes simplex virus shedding and plasma human immunodeficiency virus RNA levels in coinfected women. Clin Infect Dis 33:885–890
Whitley RJ, Roizman B (2001) Herpes simplex virus infections. Lancet 357:1513–1518
Zuckerman RA (2009) The clinical spectrum of herpes simplex viremia. Clin Infect Dis 49:1302–1304
Tyler KL (2004) Herpes simplex virus infections of the central nervous system: encephalitis and meningitis, including Mollaret’s. Herpes 11(Suppl 2):57A–64A
Mark KE, Wald A, Magaret AS et al (2008) Rapidly cleared episodes of herpes simplex virus reactivation in immunocompetent adults. J Infect Dis 198:1141–1149
Wald A, Ericsson M, Krantz E, Selke S, Corey L (2004) Oral shedding of herpes simplex virus type 2. Sex Transm Infect 80:272–276
Glenny AM, Fernandez Mauleffinch LM, Pavitt S, Walsh T (2009) Interventions for the prevention and treatment of herpes simplex virus in patients being treated for cancer. Cochrane Database Syst Rev 1, CD006706
Stoopler ET (2005) Oral herpetic infections (HSV 1–8). Dent Clin North Am 49:15–29
Acknowledgement
Research grant (no. 56/7/2007/BMS) from Indian Council of Medical Research, New Delhi is gratefully acknowledged.
Conflict of interest
The corresponding author (DB) has full control of all the primary data. They agree to allow the journal to review the data if requested. None of the authors have a financial relationship with the organization that sponsored the research.
Source of funding
Indian Council of Medical Research, New Delhi.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aggarwal, R., Bansal, D., Naru, J. et al. HSV-1 as well as HSV-2 is frequent in oral mucosal lesions of children on chemotherapy. Support Care Cancer 22, 1773–1779 (2014). https://doi.org/10.1007/s00520-014-2152-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-014-2152-0