Abstract
Purpose
Chemotherapy-induced nausea and vomiting (CINV), common adverse events of chemotherapy, may be associated with considerable healthcare resource utilization. This study was conducted to describe CINV-associated healthcare visits and costs following a first cycle of highly or moderately emetogenic chemotherapy (HEC or MEC).
Methods
This retrospective cohort study used the Premier Perspective™ Database to identify adult patients who received their first HEC or MEC and at least one antiemetic agent from 2003 to 2007 at US hospital-based outpatient facilities. Hospital visits with a CINV-related ICD-9 diagnosis were included from the chemotherapy administration date to 30 days later or 1 day before the second chemotherapy, whichever was first. CINV costs were hospital-reported costs.
Results
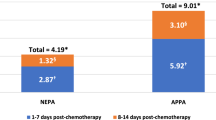
Of 19,139 patients (HEC, 16%; MEC, 84%), mean (SD) age was 59 (14) years; 59% were female; 66% were white. CINV prophylaxis included 5-HT3 antagonists (85%), dexamethasone (76%), and NK-1 antagonists (2%). Overall, 13.8% of patients had a CINV-associated visit (HEC, 18%; MEC, 13%): 0.2% for acute CINV (day of chemotherapy, excluding chemotherapy administration visit) and 13.7% for delayed CINV. CINV-associated visits included inpatient (IP, 64%), outpatient (OP, 26%), and emergency room (ER, 10%) visits. Mean (SD) costs of CINV visits were $5,299 ($6,639); for IP, $7,448 ($7,271); OP, $1,494 ($2,172); and ER, $918 ($1,071). Mean per-patient CINV-associated costs across all patients were $731 ($3,069). Sensitivity analysis excluding visits where CINV was a secondary diagnosis code resulted in a CINV incidence of 4.4%, a mean CINV visit cost of $4,043, and a mean per-patient CINV-associated cost across all patients of $176.
Conclusions
CINV visits in the first HEC or MEC cycle were common and costly, especially inpatient hospitalizations in the delayed phase. Strategies to reduce CINV in the delayed phase could reduce healthcare utilization and costs.

Similar content being viewed by others
References
Kris MG, Hesketh PJ, Somerfield MR, Feyer P, Clark-Snow R, Koeller JM, Morrow GR, Chinnery LW, Chesney MJ, Gralla RJ, Grunberg SM (2006) American Society of Clinical Oncology guideline for antiemetics in oncology: update 2006. J Clin Oncol 24:2932–2947
National Comprehensive Cancer Network (2009) Clinical practice guidelines in oncology. Antiemesis. V.1. http://www.nccn.org/professionals/physician_gls/PDF/antiemesis.pdf. Accessed 15 July 2009
Multinational Association of Supportive Care in Cancer (MASCC). Antiemetic guidelines. http://www.mascc.org/mc/page.do?sitePageId=88041&orgId=mascc. Accessed 15 July 2009
Ihbe-Heffinger A, Ehlken B, Bernard R, Berger K, Peschel C, Eichler HG, Deuson R, Thodtmann J, Lordick F (2004) The impact of delayed chemotherapy-induced nausea and vomiting on patients, health resource utilization and costs in German cancer centers. Ann Oncol 15:526–536
Molassiotis A, Saunders MP, Valle J, Wilson G, Lorigan P, Wardley A, Levine E, Cowan R, Loncaster J, Rittenberg C (2008) A prospective observational study of chemotherapy-related nausea and vomiting in routine practice in a UK cancer centre. Support Care Cancer 16:201–208
Glaus A, Knipping C, Morant R, Bohme C, Lebert B, Beldermann F, Glawogger B, Ortega PF, Husler A, Deuson R (2004) Chemotherapy-induced nausea and vomiting in routine practice: a European perspective. Support Care Cancer 12:708–715
Cohen L, de Moor CA, Eisenberg P, Ming EE, Hu H (2007) Chemotherapy-induced nausea and vomiting: incidence and impact on patient quality of life at community oncology settings. Support Care Cancer 15:497–503
Bloechl-Daum B, Deuson RR, Mavros P, Hansen M, Herrstedt J (2006) Delayed nausea and vomiting continue to reduce patients’ quality of life after highly and moderately emetogenic chemotherapy despite antiemetic treatment. J Clin Oncol 24:4472–4478
Grunberg SM, Deuson RR, Mavros P, Geling O, Hansen M, Cruciani G, Daniele B, De Pouvourville G, Rubenstein EB, Daugaard G (2004) Incidence of chemotherapy-induced nausea and emesis after modern antiemetics. Cancer 100:2261–2268
Ballatori E, Roila F, Ruggeri B, Betti M, Sarti S, Soru G, Cruciani G, Di Maio M, Andrea B, Deuson RR (2007) The impact of chemotherapy-induced nausea and vomiting on health-related quality of life. Support Care Cancer 15:179–185
Morrow GR, Roscoe JA, Hickok JT, Stern RM, Pierce HI, King DB, Banerjee TK, Weiden P (1998) Initial control of chemotherapy-induced nausea and vomiting in patient quality of life. Oncology (Williston Park) 12:32–37
Kris MG (2003) Why do we need another antiemetic? Just ask. J Clin Oncol 21:4077–4080
Liau CT, Chu NM, Liu HE, Deuson R, Lien J, Chen JS (2005) Incidence of chemotherapy-induced nausea and vomiting in Taiwan: physicians’ and nurses’ estimation vs. patients’ reported outcomes. Support Care Cancer 13:277–286
Ballatori E, Roila F (2003) Impact of nausea and vomiting on quality of life in cancer patients during chemotherapy. Health Qual Life Outcomes 1:46
Ballatori E, Roila F, Ruggeri B, Porrozzi S, Iannopollo M, Soru G, Cruciani G, Daniele B, Locatelli MC, Pellissier J, Deuson R (2007) The cost of chemotherapy-induced nausea and vomiting in Italy. Support Care Cancer 15:31–38
Tina Shih YC, Xu Y, Elting LS (2007) Costs of uncontrolled chemotherapy-induced nausea and vomiting among working-age cancer patients receiving highly or moderately emetogenic chemotherapy. Cancer 110:678–685
Roila F, Hesketh PJ, Herrstedt J, Antiemetic Subcommittee of the Multinational Association of Supportive Care in Cancer (2006) Prevention of chemotherapy- and radiotherapy-induced emesis: results of the 2004 Perugia International Antiemetic Consensus Conference. Ann Oncol 17:20–28
Mosteller RD (1987) Simplified calculation of body-surface area. N Engl J Med 317:1098
Weiner MG, Livshits A, Carozzoni C, McMenamin E, Gibson G, Loren A, Hennessy S (2003) Derivation of malignacy status from ICD-9 codes. American Medical Informatics Association 2003 Symposium Proceedings: 1050
Hesketh PJ (2008) Chemotherapy-induced nausea and vomiting. N Engl J Med 358:2482–2494
Molassiotis A, Fernadez-Ortega P, Pud D, Ozden G, Scott JA, Panteli V, Margulies A, Browall M, Magri M, Selvekerova S, Madsen E, Milovics L, Bruyns I, Gudmundsdottir G, Hummerston S, Ahmad AM, Platin N, Kearney N, Patiraki E (2005) Use of complementary and alternative medicine in cancer patients: a European survey. Ann Oncol 16:655–663
Lachaine J, Yelle L, Kaizer L, Dufour A, Hopkins S, Deuson R (2005) Chemotherapy-induced emesis: quality of life and economic impact in the context of current practice in Canada. Support Cancer Ther 2:181–187
Stewart DJ, Dahrouge S, Coyle D, Evans WK (1999) Costs of treating and preventing nausea and vomiting in patients receiving chemotherapy. J Clin Oncol 17:344–351
Moore S, Tumeh J, Wojtanowski S, Flowers C (2007) Cost-effectiveness of aprepitant for the prevention of chemotherapy-induced nausea and vomiting associated with highly emetogenic chemotherapy. Value Health 10:23–31
Acknowledgments
Editorial assistance was provided by Elizabeth V Hillyer with the financial support of Merck & Co., Inc.
Disclosures
Thomas Burke and Tami Wisniewski are employees of Merck & Co., Inc. Frank Ernst is an employee of Premier Research Services.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Burke, T.A., Wisniewski, T. & Ernst, F.R. Resource utilization and costs associated with chemotherapy-induced nausea and vomiting (CINV) following highly or moderately emetogenic chemotherapy administered in the US outpatient hospital setting. Support Care Cancer 19, 131–140 (2011). https://doi.org/10.1007/s00520-009-0797-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-009-0797-x