Abstract
Purpose
Certain patients may be unwilling to accept blood products for religious reasons. In this study, we have assessed the clinical cancer treatment outcomes of Jehovah’s Witnesses (JW) cancer patients in order to identify the risks associated with their treatment, as well as their transfusion needs.
Methods
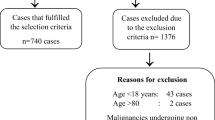
We analyzed 77 cases of histologically confirmed cancer patients (JW) from January 2001 to April 2008.
Results
The median age of the patients was 59 years (range, 8–83 years). The most common primary site was the stomach (20.8%), followed by the breast (14.3%), and colorectal region (11.7%). Operations were performed on 44 patients (89.8%). Changes in complete blood count profiles after operation were detected in the patients’ hemoglobin (mean ± SD; 12.7 ± 2.1 g/dL to 10.6 ± 2.3 g/dL, P < 0.001). Twenty-six patients received adjuvant chemotherapy. Among these, 21 (80.8%) completed their planned schedule. One hundred twenty-seven cycles of palliative intravenous chemotherapy were administered to 19 patients. Granulocyte-colony stimulating factor and erythropoietin were used in 45 and 20 cycles of treatment, respectively. Grade ≥III thrombocytopenia and anemia were noted in 3.9% and 2.4% of the patients. Three- and 5-year survival rates were 80% and 70%, respectively. The most frequent cause of death was disease progression rather than bleeding.
Conclusions
Bloodless cancer operation and chemotherapy were not accompanied by serious complications. A few cases of palliative chemotherapy also required transfusions. A prospective cohort study group will need to be used to determine precisely the safety of bloodless cancer treatment and the efficacy of transfusion alternatives.


Similar content being viewed by others
References
Goodnough LT, Shander A, Spence R (2003) Bloodless medicine: clinical care without allogeneic blood transfusion. Transfusion 43:668–676
Schmitt S, Mailaender V, Egerer G, Leo A, Becker S, Reinhardt P, Wiesneth M, Schrezenmeier H, Ho AD, Goldschmidt H, Moehler TM (2008) Successful autologous peripheral blood stem cell transplantation in a Jehovah’s Witness with multiple myeloma: review of literature and recommendations for high-dose chemotherapy without support of allogeneic blood products. Int J Hematol 87:289–297
Brown JE, Hatton MQ, Melchers R, Goldstraw P, Coleman RE (2003) Chemotherapy, erythropoietin and bloodless surgery in a Jehovah’s Witness. Clin Oncol (R Coll Radiol) 15:371–377
Johnson PW, King R, Slevin ML, White H (1991) The use of erythropoietin in a Jehovah’s Witness undergoing major surgery and chemotherapy. Br J Cancer 63:476
Brown NM, Keck G, Ford PA (2008) Acute myeloid leukemia in Jehovah Witnesses. Leuk Lymphoma 49:817–820
Ball AM, Winstead PS (2008) Recombinant human erythropoietin therapy in critically ill Jehovah’s Witnesses. Pharmacotherapy 28:1383–1390
Monk TG (2004) Preoperative recombinant human erythropoietin in anemic surgical patients. Crit Care 8(Suppl 2):S45–S48
Mercuriali F, Inghilleri G (1998) Blood transfusion in oncologic surgery: the role of recombinant human erythropoietin (rHuEPO). Tumori 84:S3–S14
Gohel MS, Bulbulia RA, Slim FJ, Poskitt KR, Whyman MR (2005) How to approach major surgery where patients refuse blood transfusion (including Jehovah’s Witnesses). Ann R Coll Surg Engl 87:3–14
Gomez-Almaguer D, Ruiz-Arguelles G, Lozano de la Vega A, Garcia-Guajardo BM (1990) Acute leukemia in Jehovah’s Witnesses: difficulties in its management. Rev Invest Clin 42:317–320
Chigbu B, Onwere S, Kamanu C, Aluka C, Okoro O, Feyi-Waboso P, Onichakwe C (2009) Lessons learned from the outcome of bloodless emergency laparotomies on Jehovah’s Witness women presenting in the extremis with ruptured uterus. Arch Gynecol Obstet 279:469–472
Adelola OA, Ahmed I, Fenton JE (2008) Management of Jehovah’s Witnesses in otolaryngology, head and neck surgery. Am J Otolaryngol 29:270–278
Rosengart TK, Helm RE, DeBois WJ, Garcia N, Krieger KH, Isom OW (1997) Open heart operations without transfusion using a multimodality blood conservation strategy in 50 Jehovah’s Witness patients: implications for a "bloodless" surgical technique. J Am Coll Surg 184:618–629
Jabbour N, Gagandeep S, Mateo R, Sher L, Genyk Y, Selby R (2005) Transfusion free surgery: single institution experience of 27 consecutive liver transplants in Jehovah’s Witnesses. J Am Coll Surg 201:412–417
Sloan JM, Ballen K (2008) SCT in Jehovah’s Witnesses: the bloodless transplant. Bone Marrow Transplant 41:837–844
Smith SE, Toor A, Rodriguez T, Stiff P (2006) The administration of polymerized human hemoglobin (Pyridoxylated) to a Jehovah’s Witness after submyeloablative stem cell transplantation complicated by delayed graft failure. Compr Ther 32:172–175
Bengala C, Guarneri V, Ledermann J, Rosti G, Wandt H, Lotz JP, Cure JH, Orlandini C, Ferrante P, Conte PF, Demirer T (2005) High-dose chemotherapy with autologous haemopoietic support for advanced ovarian cancer in first complete remission: retrospective analysis from the Solid Tumour Registry of the European Group for Blood and Marrow Transplantation (EBMT. Bone Marrow Transplant 36:25–31
Ballen KK, Ford PA, Waitkus H, Emmons RV, Levy W, Doyle P, Stewart FM, Quesenberry PJ, Becker PS (2000) Successful autologous bone marrow transplant without the use of blood product support. Bone Marrow Transplant 26:227–229
Witzig TE, Silberstein PT, Loprinzi CL, Sloan JA, Novotny PJ, Mailliard JA, Rowland KM, Alberts SR, Krook JE, Levitt R, Morton RF (2005) Phase III, randomized, double-blind study of epoetin alfa compared with placebo in anemic patients receiving chemotherapy. J Clin Oncol 23:2606–2617
Littlewood TJ, Bajetta E, Nortier JW, Vercammen E, Rapoport B (2001) Effects of epoetin alfa on hematologic parameters and quality of life in cancer patients receiving nonplatinum chemotherapy: results of a randomized, double-blind, placebo-controlled trial. J Clin Oncol 19:2865–2874
Gabrilove JL, Cleeland CS, Livingston RB, Sarokhan B, Winer E, Einhorn LH (2001) Clinical evaluation of once-weekly dosing of epoetin alfa in chemotherapy patients: improvements in hemoglobin and quality of life are similar to three-times-weekly dosing. J Clin Oncol 19:2875–2882
Osterborg A, Brandberg Y, Molostova V, Iosava G, Abdulkadyrov K, Hedenus M, Messinger D (2002) Randomized, double-blind, placebo-controlled trial of recombinant human erythropoietin, epoetin Beta, in hematologic malignancies. J Clin Oncol 20:2486–2494
Lind M, Vernon C, Cruickshank D, Wilkinson P, Littlewood T, Stuart N, Jenkinson C, Grey-Amante P, Doll H, Wild D (2002) The level of haemoglobin in anaemic cancer patients correlates positively with quality of life. Br J Cancer 86:1243–1249
Dronca RS, Steensma DP (2008) VTE and mortality associated with erythropoiesis-stimulating agents in cancer-associated anemia. Nat Clin Pract Oncol 5:504–505
Thomas G, Ali S, Hoebers FJ, Darcy KM, Rodgers WH, Patel M, Abulafia O, Lucci JA 3rd, Begg AC (2008) Phase III trial to evaluate the efficacy of maintaining hemoglobin levels above 12.0 g/dL with erythropoietin vs above 10.0 g/dL without erythropoietin in anemic patients receiving concurrent radiation and cisplatin for cervical cancer. Gynecol Oncol 108:317–325
Wright JR, Ung YC, Julian JA, Pritchard KI, Whelan TJ, Smith C, Szechtman B, Roa W, Mulroy L, Rudinskas L, Gagnon B, Okawara GS, Levine MN (2007) Randomized, double-blind, placebo-controlled trial of erythropoietin in non-small-cell lung cancer with disease-related anemia. J Clin Oncol 25:1027–1032
Acknowledgment
This paper was supported by the Dong-A University Research Fund and the Korea Science and Engineering Foundation (KOSEF) grant funded by the Korea government (MEST; R13-2002-044-05001-0).
Conflict of Interest
The authors declare that have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oh, S.Y., Kim, SH., Kwon, HC. et al. Bloodless cancer treatment results of patients who do not want blood transfusion: single center experience of 77 cases. Support Care Cancer 18, 1341–1346 (2010). https://doi.org/10.1007/s00520-009-0759-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-009-0759-3