Abstract
Introduction
The aim of our study was to evaluate the efficacy of a single-dose palonosetron plus dexamethasone to control emesis in patients (pts) receiving HEC. Moreover, we evaluated the amount of their food intake (FI) in the week following therapy, in order to measure any reduction of calories consumption related to Chemotherapy-induced nausea and vomiting (CINV).
Methods
Patients affected with advanced cancer were treated with palonosetron 250 mcg plus dexamethasone 20 mg before HEC. Nausea, vomiting, and FI were monitored by a 7-day diary. Complete Response (CR: no vomiting and no rescue therapy) was the primary endpoint, Complete Control (CC: CR and no more than mild nausea) and the evaluation of FI were the secondary endpoints. The endpoints were evaluated during the acute (0–24 h), the delayed (25–168 h) and overall (0–168 h) phases.
Results
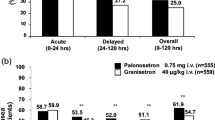
Thirty-five patients were enrolled; 85.7% and 82.9% of patients achieved CR and CC respectively, during the acute phase; 82.9% and 77.1% of patients achieved CR and CC, during the delayed phase; 80% and 77.1% of patients achieved CR and CC, during the overall phase. During the acute phase, patients with a CC without nausea had a median daily FI of 1,575 kcal, whereas patients with CC and presence of mild nausea had a median daily FI of 1,040 kcal (−535 kcal; p < 0.0001).
Conclusions
Our preliminary results confirm the efficacy of a single-dose palonosetron plus dexamethasone to prevent both acute and delayed nausea and vomiting. Moreover, the efficacy of palonosetron in nausea and vomiting control seems to warrant adequate caloric intake in these patients.


Similar content being viewed by others
References
Aapro MS, Grunberg SM, Manikhas GM, Olivares G, Suarez T, Tjulandin SA, Bertoli LF, Yunus F, Morrica B, Lordick F, Macciocchi A (2006) A phase III, double-blind, randomized trial of palonosetron compared with ondansetron in preventing chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy. Ann Oncol. 17(9):1441–1449
Aapro MS (2007) Palonosetron as an anti-emetic and anti-nausea agent in oncology. Ther Clin Risk Manag 3(6):1009–1020
Bozzetti F, SCRINIO Working Group (2009) Screening the nutritional status in oncology: a preliminary report on 1,000 outpatients. Support Care Cancer 17(3):279–284. doi:10.1007/s00520-008-0476-3
Caro MM, Laviano A, Pichard C (2007) Nutritional intervention and quality of life in adult oncology patients. Clinic Nutr 26:289–301
Eisenberg P, Figueroa-Vadillo J, Zamora R, Charu V, Hajdenberg J, Cartmell A, Macciocchi A, Grunberg S, 99-04 Palonosetron Study Group (2003) Improved prevention of moderately emetogenic chemotherapy-induced nausea and vomiting with palonosetron, a pharmacologically novel 5-HT3 receptor antagonist: results of a phase III, single-dose trial versus dolasetron. Cancer 98(11):2473–2482
Gralla R, Lichinitser M, Van Der Vegt S, Sleeboom H, Mezger J, Peschel C, Tonini G, Labianca R, Macciocchi A, Aapro M (2003) Palonosetron improves prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy: results of a double-blind randomized phase III trial comparing single doses of palonosetron with ondansetron. Ann Oncol 14(10):1570–1577
Grunberg SM, Koeller JM (2003) Palonosetron: a unique 5-HT3-receptor antagonist for the prevention of chemotherapy-induced emesis. Expert Opin Pharmacother 4(12):2297–2303
Rojas C, Stathis M, Thomas AG, Massuda EB, Alt J, Zhang J, Rubenstein E, Sebastiani S, Cantoreggi S, Snyder SH, Slusher B (2008) Palonosetron exhibits unique molecular interactions with the 5-HT3 receptor. Anesth Analg 107(2):469–478
Van Cutsem E, Arends J (2005) The causes and consequences of cancer-associated malnutrition. Eur J Oncol Nurs 9:S51–S63
Roila F, Hesketh PJ, Herrstedt J (2006) Antiemetic Subcommitte of the Multinational Association of Supportive Care in Cancer. Prevention of chemotherapy- and radiotherapy-induced emesis: results of the 2004 Perugia International Antiemetic Consensus Conference. Ann Oncol 17:20–28
de Moor C, Cunningham RS (2005) Improving the functional status of patients with cancer by more effectively preventing chemotherapy induced nausea and vomiting (CINV): a comparison of palonosetron (PALO) vs ondansetron (OND) or dolasetron (DOL). J Support Oncol 3(5 Suppl 3):25–26 PA–12.8
AIOM 2007, AIOM (Italian Association of Medical Oncology) guidelines for the treatment and prevention of neoplastic anorexia. Version December 2007.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lorusso, V., Spedicato, A., Petrucelli, L. et al. Single dose of palonosetron plus dexamethasone to control nausea, vomiting and to warrant an adequate food intake in patients treated with highly emetogenic chemotherapy (HEC). Preliminary results. Support Care Cancer 17, 1469–1473 (2009). https://doi.org/10.1007/s00520-009-0611-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-009-0611-9