Abstract
Objective
The objective of this study was to determine the efficacy of palonosetron combined with dexamethasone in prevention of acute and delayed chemotherapy-induced nausea and vomiting (CINV) in patients receiving multiple-day chemotherapy and the efficacy of a second dose of palonosetron in treating breakthrough emesis.
Materials and methods
Forty-six patients treated with multiple-day chemotherapy for hematologic malignancies received palonosetron as prophylaxis for CINV on the first day of chemotherapy and dexamethasone throughout the entire period of chemotherapy. If breakthrough emesis occurred, a second dose of palonosetron was administered after 72 h following the first administration. The results were retrospectively compared to group of patients with similar clinical characteristics undergoing similar multiple-day chemotherapy. This group had received single-dose ondansetron as CINV prophylaxis on the first day of chemotherapy plus dexamethasone throughout the entire period of chemotherapy and metoclopramide for breakthrough emesis.
Results
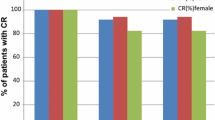
One hundred eighty and 173 chemotherapy cycles were administered in the palonosetron and ondansetron groups, respectively. Nausea and vomiting were absent in 80% of patients of the palonosetron group and 60% of the control group (p < 0.05). In the palonosetron group, 67% of patients who experienced CINV were successfully rescued by a second dose of palonosetron, while in the ondansetron group, only 22% showed a no CINV after metoclopramide treatment (p = 0.04).
Conclusions
The present results appear to be encouraging in terms of complete prophylaxis of CINV and treatment of breakthrough emesis in the setting of multiple-day chemotherapy.
Similar content being viewed by others
References
Aapro MS, Thuerlimann B, Sessa C et al (2003) A randomized double-blind trial to compare the clinical efficacy of granisetron with metoclopramide, both combined with dexamethasone in the prophylaxis of chemotherapy-induced delayed emesis. Ann Oncol 14:291–297. doi:10.1093/annonc/mdg075
Aapro M, Grunberg S, Manikhas G et al (2006) A phase III, double-blind, randomized trial of palonosetron compared with ondansetron in preventing chemotherapy-induced nausea and vomiting following higly emetogenic chemotherapy. Ann Oncol 17:1441–1449. doi:10.1093/annonc/mdl137
Abbott B, Ippoliti C, Bruton J et al (1999) Antiemetic efficacy of granisetron plus dexamethasone in bone marrow transplant patients receiving chemotherapy and total body irradiation. Bone Marrow Transplant 23:265–269. doi:10.1038/sj.bmt.1701565
Ballen KK, Hesketh AM, Heyes C et al (2001) Prospective evaluation of antiemetic outcome following high-dose chemotherapy with hematopoietic stem cell support. Bone Marrow Transplant 28:1061–1066. doi:10.1038/sj.bmt.1703280
Baltzer L, Pisters KMW, Kris MG et al (1993) High dose ondansetron plus dexamethasone for prevention of nausea and vomiting with multiple-day cisplatin chemotherapy. Proc Am Soc Clin Oncol 12:462 (abstract 462)
de Boer-Dennett M, de Witt R, Schmitz PI et al (1997) Patient percepitions of site effects of chemotherapy: the influence of the 5HT3 antagonists. Br J Cancer 76:1055–1061
Einhorn LH, Rapoport B, Koeller J et al (2005) Antiemetic therapy for multiple-day chemotherapy and high-dose chemotherapy with stem cell transplant: review and consensus statement. Support Care Cancer 13:112–116. doi:10.1007/s00520-004-0704-4
Einhorn LH, Brames MJ, Dreicer R et al (2007) Palonosetron plus dexamethasone for prevention of chemotherapy-induced nausea and vomiting in patients receiving multiple-day cisplatin chemotherapy for germ cell cancer. Support Care Cancer 15:1293–1300. doi:10.1007/s00520-007-0255-6
Eisenberg P, Figueroa-Vadillo J, Zamora R et al (2003) Improved prevention of moderately emetogenic chemotherapy-induced nausea and vomiting with palonosetron, a pharmacologically novel 5-HT3 receptor antagonist: results of a phase III, single dose trial versus dolasetron. Cancer 98:2473–2482. doi:10.1002/cncr.11817
Fauser AA, Pizzocarro G, Schuller J et al (2000) A double-blind, randomised, parallel study comparing intravenous dolasetron plus dexamethasone and intravenous dolasetron alone for the management of fractionated cisplatin-related nausea and vomiting. Support Care Cancer 8:49–54
Fox SM, Einhorn LH, Cox E et al (1993) Ondansetron versus ondansetron, dexamethasone, and chlopromazine in the prevention of nausea and vomiting associated with multiple-day cisplatin chemotherapy. J Clin Oncol 11:2391–2395
Fox-Geiman MP, Fisher SG, Kiley K et al (2001) Double-blind comparative trial of oral ondansetron versus IV ondansetron in the prevention of nausea and vomiting associated with highly emetogenic preparative regimens prior to stem cell transplantation. Biol Blood Marrow Transplant 7:596–603. doi:10.1053/bbmt.2001.v7.pm11760147
Geling O, Eichler H (2005) Should 5-hydroxytryptamine-3 receptor antagonists be administered beyond 24 hours after chemotherapy to prevent delayed emesis? Systematic re-evaluation of clinical evidence and drug cost implications. J Clin Oncol 23:1289–1294. doi:10.1200/JCO.2005.04.022
Goedhals L, Heron JF, Kleisbauer JP et al (1998) Control of delayed nausea and vomiting with granisetron plus dexamethasone or dexamethasone alone in patients receiving highly emetogenic chemotherapy: a double-blind, placebo-controlled comparative study. Ann Oncol 9:661–666. doi:10.1023/A:1008256115221
Gralla R, Lichinitser M, Van Der Vegt S et al (2003) Palonosetron improves prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy: results of a double-blind randomized phase III trial comparing single doses of palonosetron with ondansetron. Ann Oncol 14:1570–1577. doi:10.1093/annonc/mdg417
Herrstedt J, Koeller JM, Roilla F et al (2005) Acute emesis: moderately emetogenic chemotherapy. Support Care Cancer 13:97–103. doi:10.1007/s00520-004-0701-7
Hesketh PJ (2004) New treatment options for chemotherapy-induced nausea and vomiting. Support Care Cancer 12:550–554
Hesketh PJ, Kris MG, Grunberg SM et al (1997) Proposal for classifying the acute emetogenicity of cancer chemotherapy. J Clin Oncol 15:103–109
Italian Group for Antiemetic Research (2000) Dexamethasone alone or in combination with ondansetron for the prevention of delayed nausea and vomiting induced by chemotherapy. N Engl J Med 342:1554–1559. doi:10.1056/NEJM200005253422102
Kris MG, Hesketh PJ, Herrstedt J et al (2005) Consensus proposals for the prevention of acute and delayed vomiting and nausea following high-emetic-risk chemotherapy. Support Care Cancer 13:85–96. doi:10.1007/s00520-004-0699-x
Kris MG, Hesketh PJ, Somerfield MR et al (2006) American Society of Clinical Oncology guideline for antiemetics in oncology: update 2006. J Clin Oncol 24:2932–2947. doi:10.1200/JCO.2006.06.9591
Matsuoka S, Okamoto S, Watanabe R et al (2003) Granisetron plus dexamethasone versus granisetron alone in the prevention of vomiting induced by conditioning for stem cell transplantation: a prospective randomized study. Int J Hematol 77:86–90
Navari RM (2007) Prevention of emesis from multiple-day and high-dose chemotherapy regimens. J Natl Compr Canc Netw 5:51–59
Noble A, Bremer K, Goedhals L et al (1994) A double-blind, randomised crossover comparison of granisetron and ondansetron in 5-day fractionated chemotherapy: assessment of efficacy, safety, and patient preference. The Granisetron Study Group. Eur J Cancer 30:1083–1088. doi:10.1016/0959-8049(94)90461-8
Osoba D, Zee B, Peter J et al (1997) Determinants of postchemotherapy nausea and vomting in patient with cancer. J Clin Oncol 15:116–123
Osoba D, Zee B, Warr D et al (1997) Effect of post-chemotherapy nausea and vomiting on health related quality of life. Support Care Cancer 5:307–313. doi:10.1007/s005200050078
Pater JL, Lofters WS, Zee B et al (1997) The role of the 5-HT3 antagonist ondansetron and dolansetron in the control of delayed onset nausea and vomiting in patients receiving moderately emetogenic chemotherapy. Ann Oncol 8:181–185. doi:10.1023/A:1008247830641
Perez EA, Tiemeier T, Solberg LA (1999) Antiemetic therapy for high-dose chemotherapy with transplantation: report of a retrospective analysis of a 5HT3 regimen and literature review. Support Care Cancer 7:413–424. doi:10.1007/s005200050302
Roila F, Warr D, Clarck-Snow RA et al (2005) Delayed emesis: moderately emetogenic chemotherapy. Support Care Cancer 13:104–108. doi:10.1007/s00520-004-0700-8
Rojas C, Stathis M, Thomas A (2007) Palonosetron in contrast to Ondansetron and Granisetron, exhibits two-site binding to the 5-HT3 receptor and causes long lasting functional inhibition even after dissociation. Supportive Care Cancer 15:686 (abstract P017)
Sledge GW Jr, Einhorn L, Nagy C, House K (1992) Phase III double-blind comparison of intravenous ondansetron and metoclopramide for patients receiving multiple-day cisplatin based chemotherapy. Cancer 70:2524–2528. doi:10.1002/1097-0142(19921115)70:10<2524::AID-CNCR2820701022>3.0.CO;2-Z
Walsh T, Morris AK, Holle LM et al (2004) Granisetron vs ondansetron for prevention of nausea and vomiting in hematopoietic stem cell transplant patients: results of a prospective, double-blind, randomized trial. Bone Marrow Transplant 34:963–968. doi:10.1038/sj.bmt.1704714
Wong EH, Clark R, Leung E et al (1995) The interaction of RS 25259-197, a potent and selective antagonist, with 5-HT3 receptors, in vitro. Br J Pharmacol 114:851–859
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Musso, M., Scalone, R., Bonanno, V. et al. Palonosetron (Aloxi®) and dexamethasone for the prevention of acute and delayed nausea and vomiting in patients receiving multiple-day chemotherapy. Support Care Cancer 17, 205–209 (2009). https://doi.org/10.1007/s00520-008-0510-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-008-0510-5