Abstract
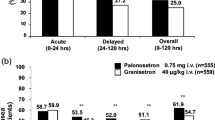
Cancer patients undergoing bone marrow transplantation (BMT) experience severe nausea and vomiting associated with high-dose chemotherapy agents; these emetic symptoms are compounded by total body irradiation used in many conditioning regimens. This paper reviews clinical experience with the 5-HT3 receptor antagonist granisetron, both as a single agent and in combination with other anti-emetics, in patients undergoing BMT and peripheral blood stem cell transplantation (PBSCT). Clinical studies demonstrate the efficacy (47–61% with no vomiting and no worse than mild nausea) and tolerability of granisetron. Its long half-life and duration of action may be responsible for its effective 24 h control of nausea and vomiting in BMT patients.



Similar content being viewed by others
References
Abang AM, Takemoto MH, Pham T, Mandanas RA, Roy V, Selby GB et al (2000) Efficacy and safety of oral granisetron versus i.v. granisetron in patients undergoing peripheral blood progenitor cell and bone marrow transplantation. Anticancer Drugs 11: 137–142
Abbott B, Ippoliti C, Bruton J, Neumann J, Whaley R, Champlin R (1999) Antiemetic efficacy of granisetron plus dexamethasone in bone marrow transplant patients receiving chemotherapy and total body irradiation. Bone Marrow Transplant 23: 265–269
Abbott B, Ippoliti C, Hecth D, Bruton J, Whaley B, Champlin R (2000) Granisetron (Kytril) plus dexamethasone for antiemetic control in bone marrow transplant patients receiving highly emetogenic chemotherapy with or without total body irradiation. Bone Marrow Transplant 25: 1279–1283
Applegate GL, Mittal BB, Kletzel M, Morgan E, Johnson P, Danner K et al (1998) Outpatient total body irradiation prior to bone marrow transplantation in pediatric patients: a feasibility analysis. Bone Marrow Transplant 21: 651–652
Belkacémi Y, Ozsahin M, Pène F, Rio B, Sutton L, Laporte J-P et al (1996) Total body irradiation prior to bone marrow transplantation: efficacy and safety of granisetron in the prophylaxis and control of radiation-induced emesis. Int J Radiat Oncol Biol Phys 36: 77–82
Bloomer JC, Baldwin SJ, Smith GJ et al (1994) Characterisation of the cytochrome P450 enzymes involved in the in vitro metabolism of granisetron. Br J Clin Pharmacol 38(6): 273–298
Blower PR. Granisetron: relating pharmacology to clinical efficacy. Supp Care Cancer 2003 11: 93–100
Bubalo J, Seelig F, Karbowicz S, Maziarz RT (2001) Randomized open-label trial of dolasetron for the control of nausea and vomiting associated with high-dose chemotherapy with haematopoietic stem cell transplantation. Biol Blood Marrow Transplant 7: 439–445
Dey B, Sykes M, Spitzer TR (1998) Outcomes of recipients of both bone marrow and solid organ transplants. A review. Medicine 77: 355–369
Fox-Geiman M, Fisher SG, Kiley K, McLean M, Fletcher-Gonzalez D, Roczniak L et al (1999) Double-blind randomized comparison of oral granisetron and i.v. ondansetron for regimen-related nausea and vomiting (N/V) in patients undergoing stem cell transplants. Proc Am Soc Clin Oncol 18: 592a
Frakes LA, Brehm TL, Kosty MP, Miller WE, McMillan RL, Mason J et al (1997) An all oral antiemetic regimen for patients undergoing high-dose chemotherapy with peripheral blood stem cell transplant. Bone Marrow Transplant 20: 473–478
Gilbert CJ et al (1998) Pharmacokinetic interaction between ondansetron and cyclophosphamide during high-dose chemotherapy for breast cancer. Cancer Chemother Pharmacol 42:497–503
Hunter AE, Prentice HG, Pothecary K, Coumar A, Collis C, Upward J et al (1991) Granisetron, a selective 5-HT3 receptor antagonist, for the prevention of radiation induced emesis during total body irradiation. Bone Marrow Transplant 7: 439–441
Imamura R, Voegels R, Sperandio, F, Sennes LU, Silva R, Butugan O et al (1999) Microbiology of sinusitis in patients undergoing bone marrow transplantation. Otolaryngol Head Neck Surg 120: 279–282
Kaiser R, Sezer O, Papies A et al (2002) Patient-tailored antiemetic treatment with 5-hydroxytryptamine type 3 receptor antagonists according to cytochrome P-450 2D6 genotypes. J Clin Oncol 20: 280–511
Kalaycio M, Mendez Z, Pohlman B, Overmoyer B, Boparai N, Jones E et al (1998) Continuous-infusion granisetron compared to ondansetron for the prevention of nausea and vomiting after high-dose chemotherapy. J Cancer Res Clin Oncol 124: 265–269
Lacerda JF, Martins C, Carmo JA, Lourenco ME, Araujo Pereira ME, Rodrigues A et al (2000) Randomized trial of ondansetron, granisetron, and tropisetron in the prevention of acute nausea and vomiting. Transplant Proc 32: 2680–2681
Lawrence CC, Gilbert CJ, Peters WP (1996) Evaluation of symptom distress in a bone marrow transplant outpatient environment. Ann Pharmacother 30: 941–945
Lehoczky O (1999) About the antiemetic effectivity of granisetron in chemotherapy-induced acute emesis: a comparison of results with intravenous and oral dosing. Neoplasma 46(2): 73–79
Mihelic RA, Walton SM, Hutcherson DA, Knoche AJ (2000) Evaluation of single-dose oral ondansetron vs oral granisetron for prevention of chemotherapy-induced nausea and vomiting (NV) due to high-dose chemotherapy. Proc Am Soc Clin Oncol 19: 631a
Nevo S, Vogelsang GB (2001) Acute bleeding complications in patients after bone marrow transplantation. Curr Opin Hematol 8: 319–325
Okamoto S, Takahashi S, Tanosaki R, Sakamaki H, Onozawa Y, Oh H et al (1996) Granisetron in the prevention of vomiting induced by conditioning for stem cell transplantation: a prospective randomized study. Bone Marrow Transplant 17: 679–683
Orchard PJ, Rogesheske J, Burns L, Rydholm N, Larson H, DeFor TE et al (1999) A prospective randomized trial of the anti-emetic efficacy of ondansetron and granisetron during bone marrow transplantation. Biol Blood Marrow Transplant 5: 386–393
Perez EA, Tiemeier T, Solberg LA (1999) Antiemetic therapy for high-dose chemotherapy with transplantation: report of a retrospective analysis of a 5-HT3 regimen and literature review. Support Care Cancer 7: 413–424
Prentice HG, Cunningham S, Gandhi L, Cunningham J, Collis C, Hamon MD (1995) Granisetron in the prevention of irradiation-induced emesis. Bone Marrow Transplant 15: 445–448
Prentice HG, Kibbler K, Prentice AG (2000) Towards a targeted, risk based antifungal strategy in neutropenic patients. Br J Haematol 110(2): 273–284
Roy V, Ochs L, Weisdorf D (1997) Late infections following allogeneic bone marrow transplantation: suggested strategies for prophylaxis. Leuk Lymphoma 26: 115
Shibuya TY, Momin F, Abella E, Jacobs JR, Karanes C, Ratanatharathorn V et al (1995) Sinus disease in the bone marrow transplant population: incidence, risk factors, and complications. Otolaryngol Head Neck Surg 113: 705–711
Slaby J, Trneny M, Prochazka B, Klener P (2000) Antiemetic efficacy of three serotonin antagonists during high-dose chemotherapy and autologous stem cell transplantation in malignant lymphoma. Neoplasma 47: 319–322
Spitzer TR, Friedman CJ, Bushnell W, Frankel SR, Raschko J (2000) Double-blind, randomized, parallel-group study on the efficacy and safety of oral granisetron and oral ondansetron in the prophylaxis of nausea and vomiting in patients receiving hyperfractionated total body irradiation. Bone Marrow Transplant 26: 203–210
Stewart JR, Fajardo LF, Gillette SM, Constine LS (1995) Radiation injury to the heart. Int J Radiat Oncol Biol Phys 31: 1205–1211
Acknowledgement
Thanks to TMG Healthcare Communications Ltd., for assistance in the preparation of this manuscript. This manuscript was supported by Hoffmann-La Roche.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Prentice, H.G. Granisetron in the control of nausea and vomiting associated with bone marrow transplantation: a review of its efficacy and tolerability. Support Care Cancer 11, 501–508 (2003). https://doi.org/10.1007/s00520-003-0480-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-003-0480-6