Summary
Renal sympathetic denervation (RDN) is an interventional supplement to medical treatment in patients with arterial hypertension. While the first sham-controlled trial, SYMPLICITY HTN‑3 was neutral, with improved procedural details, patient selection and follow-up, recent randomized sham-controlled trials of second-generation devices show a consistent blood pressure lowering effect of RDN, as compared to sham controls. These new data and the recent U.S. Food and Drug Administration (FDA) premarket approval of two RDN devices are the basis for the present recommendations update.
This joint position paper from the Austrian Society of Hypertension, together with the Austrian Society of Nephrology and the Working Group of Interventional Cardiology from the Austrian Society of Cardiology includes an overview about the available evidence on RDN and gives specific recommendations for the work-up, patient selection, pretreatment, procedural management and follow-up in patients undergoing RDN in Austria. Specifically, RDN may be used in clinical routine care, together with lifestyle measures and antihypertensive drugs, in patients with resistant hypertension (i.e. uncontrolled blood pressure on 3 antihypertensive drugs) and in those with uncontrolled hypertension, after adequate work-up, if institutional, patient-related and procedural conditions are fulfilled.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The following recommendations should guide Austrian physicians in the use of renal sympathetic denervation (RDN) in patients with different scenarios of arterial hypertension. This is an update of the previous guidelines from the Austrian Society of Hypertension from 2014 [1], as new clinical evidence about the efficacy and safety evolved [2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18], a U. S. Federal Food and Drug Administration (FDA) premarket approval for two devices was released [19, 20] and new guidance (clinical practice guidelines, position papers, consensus statements) from the European Society of Cardiology and the European Society of Hypertension were recently published [21,22,23,24].
History of RDN
The concept of RDN stems from the fact that increased sympathetic drive is a well-known key driver in systemic arterial hypertension [25]. As early as the 1930s, a surgical procedure known as thoracolumbar splanchnicectomy was developed for patients with severe forms of arterial hypertension and showed the effect of blood pressure lowering by sympathectomy [26, 27].
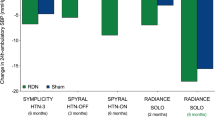
The goal of RDN is the denervation of sympathetic fibers in the adventitia of the renal arterial vasculature. In the early 2000s minimally invasive catheter-based endovascular systems were developed that facilitate the ablation of renal sympathetic nerves with high efficacy and a low complication rate. The first hype of RDN started after the publication of the initial feasibility study in 2009 [28] and the first controlled study SYMPLICITY-HTN 2 in 2010 [2]. The latter had a randomized design and found a staggering reduction of office blood pressure (BP) by 32/12 mm Hg after 6 months, while there was no difference in the control group (+1/0 mm Hg). After clinical certification the procedure was included into daily clinical care in Austria and other countries. The Austrian RDN registry with up to 300 patient cases found similar reductions in office BP compared to SYMPLICITY HTN 2 [29] and recommendations from the Austrian Society of Hypertension were published in 2012 and 2014 [1, 30]. The enthusiasm for RDN was dampened after publication of the first large sham-controlled study SYMPLICITY HTN‑3 in 2014, which found merely no effect of RDN, i.e., similar reductions of BP in the RDN and in the sham groups (Table 1). These results led to a stop in the use and reimbursement for the procedure in many countries. The European Society of Cardiology (ESC)/European Society of Hypertension (ESH) guidelines for the management of hypertension published in 2018 did not recommend RDN outside of clinical trials at all (class III indication) [31].
Later studies found regression to the mean, asymmetric data handling and a motivation towards better adherence to antihypertensive drugs in the RDN group to be the main drivers for positive results of the first studies [32]. These considerations, based on results from the SYMPLICITY-HTN 3 study and other first-generation sham-controlled clinical trials, led to a complete rethinking of procedural and patient-related aspects of RDN. The following advances were made in second-generation studies:
-
Better screening of study patients by using ambulatory BP monitoring and regular adherence checks before and after the intervention in both the RDN and the sham group.
-
Increased efficacy of RDN by using multielectrode second-generation devices, optimization of perioperative workflows and adequate training of operators.
-
Exclusion of unintended bias by use of low-noise outcome variables (such as ambulatory BP instead of office BP) and performance of a blinded sham procedure in the control group.
Second-generation trials with sham-controls now paint a homogeneous picture regarding the efficacy of RDN in patients with arterial hypertension (Table 1). In patients with mild, moderate and resistant hypertension, a consistent reduction of ambulatory BP is shown compared to sham 2–36 months after the procedure. On the basis of these data RDN is clearly a suitable option for BP lowering in selected patients with arterial hypertension, in accordance with the recently published guidelines for the management of arterial hypertension by the ESH [21].
Methods of RDN
Currently, two different physical principals are mainly used for RDN: radiofrequency (RF) ablation and ultrasound (US) ablation.
The first available system on the market was based on RF [28]. The Symplicity Renal Denervation System® (Medtronic, Minneapolis, MI, USA) has first been developed to perform point-by-point ablation around the renal artery. The first generation was time-consuming and it was deemed less efficacious because only the proximal parts could be ablated. The second generation (Symplicity Spyral, Medtronic) enables the simultaneous ablation of several points of the renal arterial system in a spiral configuration as well as an ablation of the branches of the main renal artery.
The Paradise Ultrasound Denervation System (Recor Medical Inc, Palo Alto, CA, USA) is available for RDN using unfocused US. This enables a homogeneous penetration and 360° ablation of the perivascular tissue.
Based on the favorable results from the second-generation trials outlined above, both the RF [19] and the US [20] devices received FDA premarket approval for clinical use in November 2023.
In Austria, RDN will be reimbursed in 2025 on a preliminary basis (NUB, new investigation and treatment methods, NUB—neue Untersuchungs- und Behandlungsmethoden).
An externally delivered US device has been explored but failed to show valid data on blood pressure lowering [9].
Other methods for RDN are under investigation. The RDN using perivascular alcohol injection has been explored in early studies, which showed promising results [21, 33]. A sham-controlled second-generation study has recently been published; the results were neutral [34]. Currently, no final recommendation can be given regarding this method.
Safety
The RDN is an invasive, preventive procedure that causes a clear reduction in BP as a surrogate marker but no direct reduction of cardiovascular outcomes has yet been shown. Consequently, RDN has to be a proven low-risk procedure to be accepted as an alternative to antihypertensive medication, which serves as the gold standard for treatment of arterial hypertension and is highly effective with a low risk profile.
Safety data from multiple randomized controlled trials [2,3,4,5,6,7,8,9,10,11,12,13,14,15,16] and long-term registries [29, 35] are available. If performed by experienced operators RDN can generally be considered a safe procedure. The most common complications are access site-related and occur in around 1% of cases. They can be avoided by a US-guided puncture and/or the use of a vascular closure device. The radiation dose varies and data on long-term problems due to radiation are missing as well as data on long-term negative effects due to anesthesia. The risk of contrast-associated acute kidney injury can be prevented by balanced hydration. Major vascular complications other than at the access site (i.e., dissection, perforation, intrarenal hematoma) are conceivable in theory but exceedingly rare events. In a meta-analysis of 50 trials and 5769 patients undergoing RF RDN, 7 intraprocedural dissections resulting in stent implantations were reported [36]. In general, these acute complications can be avoided with proper RDN techniques [22]. Previously feared long-term complications of RDN, such as decline in renal function and renal artery stenosis have not been shown in large observational studies and controlled trials [10, 36]. In a meta-analysis, renal arterial long-term complications (i.e., new renal artery stenoses) occurred with a similar incidence as in an untreated hypertensive population [22]. In another meta-analysis it was concluded that renal function did not change significantly for at least 9 months after RDN [37].
Efficacy of RDN
Current data show that, compared to sham-group patients, RDN leads to a moderate, albeit clinically significant reduction in 24 h SBP of 4–7 mm Hg in nearly all patient groups, from hypertension grade I to resistant hypertension. A reduction in inpatient admissions due to hypertensive emergencies was also recorded after RDN [38]; however, the procedural success is dependent on specific patient-related and procedure-related factors, which are discussed in the following sections. The durability of RDN is still a theoretical question as preclinical data suggest that reinnervation may occur theoretically 30 months after RDN in a mouse model [39]. Long-term randomized clinical trials, however, indicate a consistent BP reduction with a trend to lower BP over time for at least 3 years [13]. Recently, long-term follow-up studies of 9 and 10 years after RDN became available, indicating a long-lasting BP lowering effect of the procedure [40, 41].
Based on registry data, the reduction in BP is independent of the number and class of antihypertensive drugs at baseline [42]. Furthermore, after 3 months follow-up more patients decreased than increased the number of antihypertensive drugs.
Center qualification
First-generation randomized controlled trials of RDN showed that the procedure can lead to suboptimal results and a higher risk of complications in inexperienced centers with a low case load. For example, in the SYMPLICITY-HTN 3 study [3], low operator experience and ineffective RDN have been discussed as probable reasons for the neutral outcome [43]. Nonstandardized patient pathways can lead to inadequate patient selection without guideline-directed medical treatment or exclusion of secondary hypertension. Patients with unsuitable anatomy have to be identified preprocedurally. Furthermore, institutional expertise to adequately treat rare complications has to be present, especially in vascular surgery. The importance of institutional experience regarding RDN is highlighted in the ESH guidelines, which also recommend limiting the use of RDN to experienced centers [21].
The RDN centers should have a dedicated hypertension outpatient department, inpatient ward and departments of radiology, cardiology, nephrology, laboratory diagnostics, on-site vascular surgery and a coronary/intensive care unit. Specialization for management of complex patients with arterial hypertension is necessary and can be evidenced with dedicated diplomas (Hochdruckspezialist Österreichische Gesellschaft für Hypertensiologie, European Specialist in Hypertension ESH, Excellence Center ESH).
Multidisciplinary hypertension team (MDT)
A multidisciplinary hypertension team (MDT) can be formed that enables the informed discussion of patients suitable for RDN from various viewpoints. We strongly recommend that a hypertension specialist, certified by the ESH or a “Hochdruckspezialist” certified by the Austrian Society of Hypertension takes part in hypertension team meetings. In addition to RDN operators, a clinical cardiologist, a nephrologist and a specialist experienced in sedation (e.g., anesthesiologist, intensive care specialist) should participate. This multidisciplinary approach is also endorsed by the ESH guidelines (class I recommendation [21]).
The final decision to perform RDN should be made by a dedicated multidisciplinary hypertension team that includes at least a certified hypertension specialist, an RDN operator, a clinical cardiologist, a nephrologist and an expert on analgosedation.
RDN operators
The RDN operators should be experts in percutaneous cardiovascular interventions including access site management, radioprotection, periprocedural BP management, analgesia, and the renal arterial anatomy. We recommend that operators should first gain experience in vascular interventions before performing RDN. Furthermore, operators should receive hands-on training using a bench model of RDN and off-site attendance in an active RDN center. Proctoring of the first cases should reduce the risk of complications in operators starting to become self-dependent.
The RDN operators should have performed a sufficient number of RDN procedures with a proctor before performing an RDN procedure independently. To retain experience, operators should perform RDN procedures on a regular basis.
Patient selection
Adherence to medical treatment
Ensuring adherence to medical treatment is one of the cornerstones of initially asymptomatic diseases, such as hypertension [44]. Current guidelines for treatment of hypertension recommend the use antihypertensive polypills to increase adherence by reduction of side effects and ease of use [44]. An informed discussion with the patient about possible side effects is essential when starting antihypertensive treatment. The time of drug intake, in the morning or in the evening, may be adapted to best fit the daily life of the patient as the TIME study did not show any benefit of the evening over the morning dose administration [45]. Good adherence leads to better outcomes [46] but assessment of adherence is challenging outside clinical trials and may be very difficult to measure in the clinical routine [21]. The adherence to antihypertensive medication should be checked and discussed with the patient. In a large number of patients as evidenced for instance in all recent high-quality RDN trials, persistent and complete adherence is hard to achieve. As the primary goal is BP control, patients who are repeatedly nonadherent (if this reflects the unwillingness of the patient to take drugs) or intolerant to multiple antihypertensive drugs, can also be considered for RDN after information about the potential lack of effect and benefits and also the risks associated with the procedure. These patients may be on fewer than three drugs at the time of their selection for RDN [21, 22].
Adherence to medical treatment should be ascertained before considering RDN in patients with arterial hypertension, for instance with witnessed drug intake, laboratory drug monitoring, or monitoring of prescription refills. The results of these tests should be discussed with the patient. As BP lowering is the goal to reduce cardiovascular risk, RDN could be an option for patients unable to be fully adherent to antihypertensive drugs, for instance due to side effects, in certain conditions.
Screening for RDN and shared decision making
All patients considered for RDN have to undergo investigations, screening for secondary hypertension as recommended by international guidelines [21] and optimization of treatment at a hypertension clinic. The 24 h ambulatory BP monitoring (ABPM) is an integral part of the diagnostic work-up to exclude white-coat hypertension.
Before RDN can be considered, secondary hypertension has to be excluded, antihypertensive treatment should be optimized at a hypertension clinic, and persistence of high BP has to be evaluated using ABPM.
As RDN is an invasive procedure, available and safe oral reserve antihypertensive medications as possible alternatives, potential complications and the need to continue medical antihypertensive treatment despite the procedure in most instances, should be discussed thoroughly with the patient. In addition to individual clinical expertise and available external clinical evidence from high-quality RDN studies [47], the patient’s specific needs as well as possible intolerances to medical treatment, should be incorporated in the final decision to perform RDN.
The patient’s needs and expectations should be included in the final decision to perform RDN.
Resistant hypertension
Resistant hypertension is defined as not reaching BP targets despite treatment with at least three antihypertensive medications including one diuretic at maximum tolerated doses [44]. Patients with resistant hypertension are the best studied hypertensive population undergoing RDN and are therefore the preferred patient group. We propose the following inclusion criteria for patients undergoing RDN:
The RDN is a reasonable additional treatment option in patients with resistant hypertension and:
-
Taking at least 3 different antihypertensive medications, one of which should be a diuretic.
-
Have an average 24‑h SBP of ≥ 130 mm Hg or an average daytime SBP of ≥ 135 mm Hg in a recent 24‑h BP recording.
-
Are at least 18 years old.
-
Have an estimated glomerular filtration rate of ≥ 40 ml/min/1.73 m2 body surface area.
Mild hypertension
In patients with arterial hypertension grade I who take only few antihypertensives (usually defined as 0–1 antihypertensives), RDN may prevent the necessity of taking antihypertensives at all. Two studies found positive results of RDN in this patient population [6, 10]; however, a high number of different well-tolerated and well-studied antihypertensive medications are available for this patient population. Therefore, the panellists believe that RDN may only be considered in this patient population in selected cases after carefully evaluating benefits and harms, especially taking the individual tolerability to antihypertensive medication into account.
In patients with mild hypertension, RDN may be considered in selected cases, specifically in the presence of intolerance to several antihypertensive drug classes, considering the patient’s needs and shared decision making.
Uncontrolled hypertension with intolerance to antihypertensive drugs
Patients with uncontrolled hypertension have been included in several sham-controlled trials with improvement in BP control [8, 14]. The use of RDN may therefore be considered an option for patients with uncontrolled hypertension despite attempting lifestyle modifications and antihypertensive medication but who are either intolerant to additional medication or do not wish to be on additional medications and who are willing to undergo RDN after shared decision-making [48].
The RDN can be considered as a treatment option in patients with an eGFR > 40 ml/min/1.73 m2 who have uncontrolled BP, if drug treatment elicits serious side effects and poor quality of life.
Current European Society of Hypertension guidelines
In 2023 the European Society of Hypertension released new guidelines incorporating RDN as a treatment option in patients with an eGFR of > 40 ml/min/1.73 m2 with uncontrolled BP despite the use of antihypertensive drug combination treatment or if drug treatment would lead to serious side effects and reduced quality of life (class of recommendation II, level of evidence B) [21]. Furthermore, RDN can be considered as an additional treatment option in patients with true resistant hypertension and eGFR > 40 ml/min/1.73m2 (class of recommendation II, level of evidence B); however, RDN should only be performed in experienced specialized centers and the selection of patients undergoing RDN must incorporate shared decision making (class of recommendation I for both recommendations, expert opinion) [21].
Predictor of response to RDN
Although RDN undoubtedly lowers BP in groups of patients, the effect of the intervention in individual patients is heterogeneous, resembling the situation with different antihypertensive drug classes [49]. The topic is currently under investigation and potential candidates are heart rate [50], pulsatile hemodynamics/arterial stiffness [51], renin [52], and many others.
When not to perform RDN
Due to technical or individual considerations or absence of evidence, patients with the following factors should not undergo RDN (Fig. 1).
The RDN should not be performed in patients with the following prohibitive conditions (contraindications):
-
Unsuitable renal arterial anatomy.
-
Presence of accessory untreatable arteries.
-
Inappropriate vessel diameter.
-
Advanced renal artery atherosclerosis.
-
Renal artery stenosis.
-
Fibromuscular dysplasia.
-
Previous renal artery stenting.
-
-
Secondary hypertension.
-
Undergoing abdominal dialysis or hemodialysis.
-
Unstable clinical situations (acute coronary syndromes, acute cerebrovascular events etc.).
-
Pregnancy.
-
Age < 18 years or > 85 years.
The RDN should not be performed in patients in the following situations due to insufficient clinical evidence:
-
Severely impaired kidney function (< 40 mL/min).
-
Single functioning kidney.
-
Kidney transplant recipients.
Procedural considerations
Procedural planning and patient preparation
Adequate imaging is crucial for procedural planning and identification of potential anatomical ineligibilities.
Non-invasive renal artery imaging using either computed tomography or magnetic resonance imaging should be preferred over duplex ultrasound to identify:
-
The presence of accessory arteries.
-
Anatomical anomalies that prohibit an RDN procedure (e.g., inappropriate vessel diameter, untreated atherosclerotic or fibromuscular dysplasia, renal artery stenosis).
-
Extent of abdominal aorta/iliofemoral arteries atherothrombotic disease.
Selective renal angiography immediately before RDN remains the gold standard for identification of renal artery abnormalities.
To reduce complications all measures should be undertaken to minimize the risk of complications. This includes adequate preparation of the procedure as well as sophisticated bail-out strategies. The following recommendations are adapted from the clinical consensus statement from the ESC Council on Hypertension and the European Association of Percutaneous Cardiovascular Interventions regarding renal denervation in the management of hypertension in adults [22].
-
We recommend the establishment of a standard operating procedure that includes acute management in case of complications.
-
Continuous monitoring of vital parameters should be performed to identify complications early.
-
If applicable, antidotes of anaesthetics should be available in the catheter laboratory (e.g., naloxone and flumazenil).
-
Patients should be hydrated to euvolemia to reduce the risk of acute kidney injury.
-
Intraprocedural administration of unfractionated heparin (100 U/kg or a target ACT > 250 s) is advised.
-
Preprocedural aspirin should be administered as loading dose, followed by 100 mg daily until 1 month postprocedure. In the case of oral anticoagulant therapy, antithrombotic therapy should be tailored according to ESC guidelines for chronic coronary syndromes related to endovascular interventions [53].
Procedure
As ablation of the renal arteries is painful, patients should be sedated during the procedure by a specialist trained in sedation. Analgesia may be performed with opioids. Vital signs should be monitored and intravenous drugs for BP control should be available in the catheter laboratory.
For RDN we recommend analgosedation with low doses of opioids (e.g., fentanyl) together with sedating drugs (e.g., midazolam or propofol).
Intra-arterial nitrates are recommended preprocedurally (in the absence of hypotension). The BP should be monitored invasively and corrected when necessary. Intravenous drugs for BP control should be available in the catheter laboratory.
As a significant proportion of complications are derived from the vascular access, it should be gained with maximum caution and all available tools should be used to minimize the risk of adverse events. Radiation should be kept to a minimum. A 6 French catheter is used in RF ablation and a 7 French catheter in the US-based device.
Femoral arterial access may be performed under US guidance, if the operator is experienced to do so. Vascular closure devices should be used to reduce the risk of complications.
Modern monoplane or biplane angiographic systems should be used to reduce the radiation dose.
At the end of the procedure, angiography of the renal artery should exclude potential renal parenchymal or arterial injuries.
Follow-up and quality control
Regular follow-up of patients undergoing RDN is necessary to monitor and eventually react to changes in BP profiles or renal function. By regular follow-up long-term complications can be identified and treated earlier. At the suspicion of a late renal vascular complication, renal angiography by computed tomography or vascular ultrasound should be performed.
Centers performing RDN are responsible for adequate follow-up at 3, 6 and 12 months after the procedure and at yearly intervals thereafter, including assessment of renal function and BP.
While RDN shows consistent BP reduction in selected patients in the setting of randomized controlled trials in experienced centers, there are still limited data about the use of second-generation devices in daily clinical practice in centers with less experience. It is therefore crucial to document the efficacy and safety of RDN outside of clinical trials. This documentation furthermore serves as quality control for centers performing RDN. A national prospective registry [29, 54] will be re-established that should capture baseline, procedural and outcome data of all RDN procedures in Austria.
Procedural and outcome data of all patients undergoing RDN should be collected and included in a prospective trial, study and/or registry.
Conclusion
This position paper should be seen as guidance for physicians performing RDN in Austria. When specific conditions regarding the RDN center, the patient and the procedure are fulfilled, RDN can be a useful supplement to medical antihypertensive treatment in patients with arterial hypertension.
References
Weber T, Zweiker R, Koppelstatter C, Lambert T, Brussee H, Eber B, et al. Renale Sympathikusdenervierung 2014 in Österreich: Update der Empfehlungen der Österreichischen Gesellschaft für Hypertensiologie. Austrian J Hypertens. 2014in;18(2):54–60.
Symplicity HTNI, Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, et al. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN‑2 Trial): a randomised controlled trial. Lancet. 2010;376(9756):1903–9. https://doi.org/10.1016/S0140-6736(10)62039-9.
Bhatt DL, Kandzari DE, O’Neill WW, D’Agostino R, Flack JM, Katzen BT, et al. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370(15):1393–401. https://doi.org/10.1056/NEJMoa1402670.
Azizi M, Sapoval M, Gosse P, Monge M, Bobrie G, Delsart P, et al. Optimum and stepped care standardised antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN): a multicentre, open-label, randomised controlled trial. Lancet. 2015;385(9981):1957–65. https://doi.org/10.1016/S0140-6736(14)61942-5.
Desch S, Okon T, Heinemann D, Kulle K, Rohnert K, Sonnabend M, et al. Randomized sham-controlled trial of renal sympathetic denervation in mild resistant hypertension. Hypertension. 2015;65(6):1202–8. https://doi.org/10.1161/HYPERTENSIONAHA.115.05283.
Townsend RR, Mahfoud F, Kandzari DE, Kario K, Pocock S, Weber MA, et al. Catheter-based renal denervation in patients with uncontrolled hypertension in the absence of antihypertensive medications (SPYRAL HTN-OFF MED): a randomised, sham-controlled, proof-of-concept trial. Lancet. 2017;390(10108):2160–70. https://doi.org/10.1016/S0140-6736(17)32281-X.
Azizi M, Schmieder RE, Mahfoud F, Weber MA, Daemen J, Davies J, et al. Endovascular ultrasound renal denervation to treat hypertension (RADIANCE-HTN SOLO): a multicentre, international, single-blind, randomised, sham-controlled trial. Lancet. 2018;391(10137):2335–45. https://doi.org/10.1016/s0140-6736(18)31082-1.
Kandzari DE, Bohm M, Mahfoud F, Townsend RR, Weber MA, Pocock S, et al. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6‑month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet. 2018;391(10137):2346–55. https://doi.org/10.1016/s0140-6736(18)30951-6.
Schmieder RE, Ott C, Toennes SW, Bramlage P, Gertner M, Dawood O, et al. Phase II randomized sham-controlled study of renal denervation for individuals with uncontrolled hypertension—WAVE IV. J Hypertens. 2018;36(3):680–9. https://doi.org/10.1097/HJH.0000000000001584.
Böhm M, Kario K, Kandzari DE, Mahfoud F, Weber MA, Schmieder RE, et al. Efficacy of catheter-based renal denervation in the absence of antihypertensive medications (SPYRAL HTN-OFF MED Pivotal): a multicentre, randomised, sham-controlled tria. Lancet. 2020;395(10234):1444–51. https://doi.org/10.1016/s0140-6736(20)30554-7.
Weber MA, Kirtane AJ, Weir MR, Radhakrishnan J, Das T, Berk M, et al. The REDUCE HTN: REINFORCE: Randomized, Sham-Controlled Trial of Bipolar Radiofrequency Renal Denervation for the Treatment of Hypertension. JACC Cardiovasc Interv. 2020;13(4):461–70. https://doi.org/10.1016/j.jcin.2019.10.061.
Azizi M, Sanghvi K, Saxena M, Gosse P, Reilly JP, Levy T, et al. Ultrasound renal denervation for hypertension resistant to a triple medication pill (RADIANCE-HTN TRIO): a randomised, multicentre, single-blind, sham-controlled trial. Lancet. 2021;397(10293):2476–86. https://doi.org/10.1016/S0140-6736(21)00788-1.
Mahfoud F, Kandzari DE, Kario K, Townsend RR, Weber MA, Schmieder RE, et al. Long-term efficacy and safety of renal denervation in the presence of antihypertensive drugs (SPYRAL HTN-ON MED): a randomised, sham-controlled trial. Lancet. 2022;399(10333):1401–10. https://doi.org/10.1016/s0140-6736(22)00455-x.
Azizi M, Saxena M, Wang Y, Jenkins JS, Devireddy C, Rader F, et al. Endovascular Ultrasound Renal Denervation to Treat Hypertension: The RADIANCE II Randomized Clinical Trial. Jama : J Am Med Assoc. 2023;329(8):651–61. https://doi.org/10.1001/jama.2023.0713.
Mathiassen ON, Vase H, Bech JN, Christensen KL, Buus NH, Schroeder AP, et al. Renal denervation in treatment-resistant essential hypertension. A randomized, SHAM-controlled, double-blinded 24‑h blood pressure-based trial. J Hypertens. 2016;34(8):1639–47. https://doi.org/10.1097/HJH.0000000000000977.
Kario K, Yokoi Y, Okamura K, Fujihara M, Ogoyama Y, Yamamoto E, et al. Catheter-based ultrasound renal denervation in patients with resistant hypertension: the randomized, controlled REQUIRE trial. Hypertens Res. 2022;45(2):221–31. https://doi.org/10.1038/s41440-021-00754-7.
Kario K, Mahfoud F, Kandzari DE, Townsend RR, Weber MA, Schmieder RE, et al. Long-term reduction in morning and nighttime blood pressure after renal denervation: 36-month results from SPYRAL HTN-ON MED trial. Hypertens Res. 2023;46(1):280–8. https://doi.org/10.1038/s41440-022-01042-8.
Kandzari DE, Townsend RR, Kario K, Mahfoud F, Weber MA, Schmieder RE, et al. Safety and Efficacy of Renal Denervation in Patients Taking Antihypertensive Medications. J Am Coll Cardiol. 2023;82(19):1809–23. https://doi.org/10.1016/j.jacc.2023.08.045.
U. S. Food and Drug Administration. PMA P220026: Symplicity Spyral™ Renal Denervation System. In: https://www.accessdata.fda.gov/cdrh_docs/pdf22/P220026A.pdf.
U. S. Food and Drug Administration. PMA P220023: Paradise® Ultrasound Renal Denervation System. In: https://www.accessdata.fda.gov/cdrh_docs/pdf22/P220023A.pdf.
Mancia G, Kreutz Co-Chair R, Brunstrom M, Burnier M, Grassi G, Januszewicz A, et al. ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension Endorsed by the European Renal Association (ERA) and the International Society of Hypertension (ISH). J Hypertens. 2023; https://doi.org/10.1097/HJH.0000000000003480.
Barbato E, Azizi M, Schmieder RE, Lauder L, Bohm M, Brouwers S, et al. Renal denervation in the management of hypertension in adults. A clinical consensus statement of the ESC Council on Hypertension and the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2023;44(15):1313–30. https://doi.org/10.1093/eurheartj/ehad054.
Schmieder RE, Mahfoud F, Mancia G, Azizi M, Böhm M, Dimitriadis K, et al. European Society of Hypertension position paper on renal denervation 2021. J Hypertens. 2021;39(9):1733–41. https://doi.org/10.1097/hjh.0000000000002933.
Mahfoud F, Galle J, Schunkert H, Schmieder RE, Rump LC, Limbourg FP, et al. Kriterien der Deutschen Gesellschaft für Kardiologie – Herz- und Kreislaufforschung e. V.. (DGK), der Deutschen Hochdruckliga e. V. DHL®/Deutschen Gesellschaft für Hypertonie und Prävention und der Deutschen Gesellschaft für Nephrologie (DGfN) zur Zertifizierung von “Renale-Denervations-Zentren (RDZ)” – Update. Kardiologe. 2021;15(5):463–70. https://doi.org/10.1007/s12181-021-00492-7.
Schlaich MP, Lambert E, Kaye DM, Krozowski Z, Campbell DJ, Lambert G, et al. Sympathetic augmentation in hypertension: role of nerve firing, norepinephrine reuptake, and Angiotensin neuromodulation. Hypertension. 2004;43(2):169–75. https://doi.org/10.1161/01.HYP.0000103160.35395.9E.
Doumas M, Papademetriou V, Douma S, Faselis C, Tsioufis K, Gkaliagkousi E, et al. Benefits from treatment and control of patients with resistant hypertension. Int J Hypertens. 2010;2011:318549. https://doi.org/10.4061/2011/318549.
Smithwick RH, Thompson JE. Splanchnicectomy for essential hypertension; results in 1,266 cases. J Am Med Assoc. 1953;152(16):1501–4. https://doi.org/10.1001/jama.1953.03690160001001.
Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373(9671):1275–81. https://doi.org/10.1016/S0140-6736(09)60566-3.
Zweiker D, Lambert T, Steinwender C, Weber T, Suppan M, Brussee H, et al. Effects of Renal Denervation Documented in the Austrian National Multicentre Renal Denervation Registry. PLoS ONE. 2016;11(8):e161250. https://doi.org/10.1371/journal.pone.0161250.
Weber T, Zweiker R, Watschinger B, Gruner P, Koppelstatter C, Brandt MC, et al. Clinical application of interventional renal sympathetic denervation: recommendations of the Austrian Society of Hypertension 2012. Wien Klin Wochenschr. 2012;124(21-22):789–98. https://doi.org/10.1007/s00508-012-0257-3.
Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021–104. https://doi.org/10.1093/eurheartj/ehy339.
Howard JP, Shun-Shin MJ, Hartley A, Bhatt DL, Krum H, Francis DP. Quantifying the 3 Biases That Lead to Unintentional Overestimation of the Blood Pressure-Lowering Effect of Renal Denervation. Circ Cardiovasc Qual Outcomes. 2016;9(1):14–22. https://doi.org/10.1161/circoutcomes.115.002533.
Biffi A, Dell’Oro R, Quarti-Trevano F, Cuspidi C, Corrao G, Mancia G, et al. Effects of Renal Denervation on Sympathetic Nerve Traffic and Correlates in Drug-Resistant and Uncontrolled Hypertension: A Systematic Review and Meta-Analysis. Hypertension. 2023;80(3):659–67. https://doi.org/10.1161/HYPERTENSIONAHA.122.20503.
Pathak A, Rudolph UM, Saxena M, Zeller T, Muller-Ehmsen J, Lipsic E, et al. Alcohol-mediated renal denervation in patients with hypertension in the absence of antihypertensive medications. EuroIntervention. 2023;19(7):602–11. https://doi.org/10.4244/EIJ-D-23-00088.
Mahfoud F, Bohm M, Schmieder R, Narkiewicz K, Ewen S, Ruilope L, et al. Effects of renal denervation on kidney function and long-term outcomes: 3‑year follow-up from the Global SYMPLICITY Registry. Eur Heart J. 2019;40(42):3474–82. https://doi.org/10.1093/eurheartj/ehz118.
Townsend RR, Walton A, Hettrick DA, Hickey GL, Weil J, Sharp ASP, et al. Review and meta-analysis of renal artery damage following percutaneous renal denervation with radiofrequency renal artery ablation. EuroIntervention. 2020;16(1):89–96. https://doi.org/10.4244/EIJ-D-19-00902.
Sanders MF, Reitsma JB, Morpey M, Gremmels H, Bots ML, Pisano A, et al. Renal safety of catheter-based renal denervation: systematic review and meta-analysis. Nephrol Dial Transplant : Off Publ Eur Dial Transpl Assoc Ren Assoc. 2017;32(9):1440–7. https://doi.org/10.1093/ndt/gfx088.
Weber MA, Schmieder RE, Kandzari DE, Townsend RR, Mahfoud F, Tsioufis K, et al. Hypertension urgencies in the SPYRAL HTN-OFF MED Pivotal trial. Clin Res Cardiol. 2022;111(11):1269–75. https://doi.org/10.1007/s00392-022-02064-5.
Singh RR, McArdle ZM, Iudica M, Easton LK, Booth LC, May CN, et al. Sustained Decrease in Blood Pressure and Reduced Anatomical and Functional Reinnervation of Renal Nerves in Hypertensive Sheep 30 Months After Catheter-Based Renal Denervation. Hypertension. 2019;73(3):718–27. https://doi.org/10.1161/HYPERTENSIONAHA.118.12250.
Sesa-Ashton G, Nolde JM, Muente I, Carnagarin R, Lee R, Macefield VG, et al. Catheter-Based Renal Denervation: 9‑Year Follow-Up Data on Safety and Blood Pressure Reduction in Patients With Resistant. Hypertens Hypertens. 2023;80(4):811–9. https://doi.org/10.1161/HYPERTENSIONAHA.122.20853.
Al Ghorani H, Kulenthiran S, Recktenwald MJM, Lauder L, Kunz M, Gotzinger F, et al. 10-Year Outcomes of Catheter-Based Renal Denervation in Patients With Resistant Hypertension. J Am Coll Cardiol. 2023;81(5):517–9. https://doi.org/10.1016/j.jacc.2022.11.038.
Mahfoud F, Mancia G, Schmieder RE, Ruilope L, Narkiewicz K, Schlaich M, et al. Outcomes Following Radiofrequency Renal Denervation According to Antihypertensive Medications: Subgroup Analysis of the Global SYMPLICITY Registry DEFINE. Hypertension. 2023;80(8):1759–70. https://doi.org/10.1161/HYPERTENSIONAHA.123.21283.
Shishehbor MH, Hammad TA, Thomas G. Renal denervation: What happened, and why? Cleve Clin J Med. 2017;84(9):681–6. https://doi.org/10.3949/ccjm.84a.14129.
Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, et al. ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34(28):2159–219. https://doi.org/10.1093/eurheartj/eht151.
Mackenzie IS, Rogers A, Poulter NR, Williams B, Brown MJ, Webb DJ, et al. Cardiovascular outcomes in adults with hypertension with evening versus morning dosing of usual antihypertensives in the UK (TIME study): a prospective, randomised, open-label, blinded-endpoint clinical trial. Lancet. 2022;400(10361):1417–25. https://doi.org/10.1016/S0140-6736(22)01786-X.
Mazzaglia G, Ambrosioni E, Alacqua M, Filippi A, Sessa E, Immordino V, et al. Adherence to antihypertensive medications and cardiovascular morbidity among newly diagnosed hypertensive patients. Circulation. 2009;120(16):1598–605. https://doi.org/10.1161/CIRCULATIONAHA.108.830299.
Sackett DL, Rosenberg WM, Gray JA, Haynes RB, Richardson WS. Evidence based medicine: what it is and what it isn’t. BMJ. 1996;312(7023):71–2. https://doi.org/10.1136/bmj.312.7023.71.
Swaminathan RV, East CA, Feldman DN, Fisher ND, Garasic JM, Giri JS, et al. SCAI Position Statement on Renal Denervation for Hypertension: Patient Selection, Operator Competence, Training and Techniques, and Organizational Recommendations. J Soc Cardiovasc Angiogr Interv. 2023; https://doi.org/10.1016/j.jscai.2023.101121.
Sundstrom J, Lind L, Nowrouzi S, Hagstrom E, Held C, Lytsy P, et al. Heterogeneity in Blood Pressure Response to 4 Antihypertensive Drugs: A Randomized Clinical Trial. Jama : J Am Med Assoc. 2023;329(14):1160–9. https://doi.org/10.1001/jama.2023.3322.
Bohm M, Mahfoud F, Townsend RR, Kandzari DE, Pocock S, Ukena C, et al. Ambulatory heart rate reduction after catheter-based renal denervation in hypertensive patients not receiving anti-hypertensive medications: data from SPYRAL HTN-OFF MED, a randomized, sham-controlled, proof-of-concept trial. Eur Heart J. 2019;40(9):743–51. https://doi.org/10.1093/eurheartj/ehy871.
Weber T, Wassertheurer S, Mayer CC, Hametner B, Danninger K, Townsend RR, et al. Twenty-Four-Hour Pulsatile Hemodynamics Predict Brachial Blood Pressure Response to Renal Denervation in the SPYRAL HTN-OFF MED Trial. Hypertension. 2022;79(7):1506–14. https://doi.org/10.1161/HYPERTENSIONAHA.121.18641.
Mahfoud F, Townsend RR, Kandzari DE, Kario K, Schmieder RE, Tsioufis K, et al. Changes in Plasma Renin Activity After Renal Artery Sympathetic Denervation. J Am Coll Cardiol. 2021;77(23):2909–19. https://doi.org/10.1016/j.jacc.2021.04.044.
Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2019;41(3):407–77. https://doi.org/10.1093/eurheartj/ehz425.
Zweiker D, Lambert T, Steinwender C, Weber T, Suppan M, Brussee H, et al. Blood pressure changes after renal denervation are more pronounced in women and nondiabetic patients: findings from the Austrian Transcatheter Renal Denervation Registry. J Hypertens. 2019;37(11):2290–7. https://doi.org/10.1097/HJH.0000000000002190.
Funding
Open access funding provided by Medical University of Graz.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
C. Koppelstätter: honoraria for lectures: Medtronic. T. Weber: honoraria for lectures and advisory boards: Medtronic, Recor. D. Zweiker, K. Hohenstein, I. Lang, S. Perl, H. Bugger, M.-C. Brandt, S. Horn, R.K. Binder, B. Watschinger, M. Frick and A. Niessner declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zweiker, D., Koppelstätter, C., Hohenstein, K. et al. Renal sympathetic denervation 2024 in Austria: recommendations from the Austrian Society of Hypertension. Wien Klin Wochenschr 136 (Suppl 14), 559–569 (2024). https://doi.org/10.1007/s00508-024-02440-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00508-024-02440-3