Summary
Objective
Investigating the impact of FOXP3 (transcription factor forkhead box P3) expression on the biological behavior of esophageal squamous cell carcinoma (ESCC) and its influence on the sensitivity of ESCC cells towards cetuximab-targeted (an EGFR monoclonal antibody inhibitor) therapy.
Methods
A specifically designed recombinant FOXP3 shRNA plasmid was synthesized to target the human FOXP3 gene, and the plasmid was transfected into TE12 cells using a liposome method. Multiple assays were conducted to evaluate the effect of FOXP3 expression on ESCC cells and their response to cetuximab treatment. Proliferation activity and cetuximab sensitivity of ESCC cells were measured using the CCK‑8 assay. The invasion ability of cells was assessed using an in vitro invasion assay. Furthermore, the efficacy of cetuximab in treating ESCC was analyzed using a tumorigenesis assay in nude mice.
Results
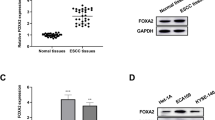
Silencing the FOXP3 gene in the TE12 cell line (shFOXP3 group) resulted in a significant reduction in FOXP3 mRNA and protein expression (p = 0.013). The shFOXP3 group exhibited slowed cell growth (p = 0.035), decreased invasion rate (p = 0.031), and increased sensitivity to cetuximab treatment (p = 0.039) compared to the control group (shNC group). In the in vivo tumorigenesis assay, the shFOXP3 group demonstrated a significant reduction in tumor volume and lung metastasis rate following cetuximab treatment (p = 0.028 and 0.007, respectively).
Conclusion
High FOXP3 expression promotes the proliferation and migration of ESCC cells, while negatively affecting their sensitivity to cetuximab-targeted therapy. Consequently, targeting FOXP3 shows potential therapeutic implications for enhancing the effectiveness of cetuximab treatment in ESCC patients.




Similar content being viewed by others
References
Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–32. https://doi.org/10.3322/caac.21338.
Jiang D, Li X, Wang H, et al. The prognostic value of EGFR overexpression and amplification in Esophageal squamous cell Carcinoma. BMC Cancer. 2015;15:377. https://doi.org/10.1186/s12885-015-1393-8.
Gu ZD, Li JY, Li M, et al. Matrix metalloproteinases expression correlates with survival in patients with esophageal squamous cell carcinoma. Am J Gastroenterol. 2005;100(8):1835–43. https://doi.org/10.1111/j.1572-0241.2005.50018.x. Erratum in: Am J Gastroenterol. 2006 Sep;101(9):2171.
Song Y, Li L, Ou Y, et al. Identification of genomic alterations in oesophageal squamous cell cancer. Nature. 2014;509(7498):91–5. https://doi.org/10.1038/nature13176.
Brenner B, Purim O, Gordon N, et al. The addition of cetuximab to preoperative chemoradiotherapy for locally advanced esophageal squamous cell carcinoma is associated with high rate of long term survival: Mature results from a prospective phase Ib/II trial. Radiother Oncol. 2019;134:74–80. https://doi.org/10.1016/j.radonc.2019.01.013.
Sakaguchi S, Vignali DA, Rudensky AY, et al. The plasticity and stability of regulatory T cells. Nat Rev Immunol. 2013;13(6):461–7. https://doi.org/10.1038/nri3464.
Luo Q, Zhang S, Wei H, et al. Roles of Foxp3 in the occurrence and development of cervical cancer. Int J Clin Exp Pathol. 2015;8(8):8717–30.
Zhu J, Li Z, Chen J, et al. A comprehensive bioinformatics analysis of FOXP3 in nonsmall cell lung cancer. Medicine. 2022;101(50):e32102. https://doi.org/10.1097/MD.0000000000032102.
Sun X, Feng Z, Wang Y, et al. Expression of Foxp3 and its prognostic significance in colorectal cancer. Int J Immunopathol Pharmacol. 2017;30(2):201–6. https://doi.org/10.1177/0394632017710415.
Zhang B, Dou Y, Xu X, et al. Endogenous FOXP3 inhibits cell proliferation, migration and invasion in glioma cells. Int J Clin Exp Med. 2015;8(2):1792–802.
Wu L, Yi B, Wei S, et al. Loss of FOXP3 and TSC1 accelerates prostate cancer progression through synergistic transcriptional and posttranslational regulation of c‑MYC. Cancer Res. 2019;79(7):1413–25. https://doi.org/10.1158/0008-5472.CAN-18-2049.
Liu C, Han J, Li X, et al. FOXP3 inhibits the metastasis of breast cancer by downregulating the expression of MTA1. Front Oncol. 2021;11:656190. https://doi.org/10.3389/fonc.2021.656190.
Wang S, Zhang Y, Wang Y, et al. Amphiregulin confers regulatory T cell suppressive function and tumor invasion via the EGFR/GSK-3β/Foxp3 axis. J Biol Chem. 2016;291(40):21085–95. https://doi.org/10.1074/jbc.M116.717892.
Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4(4):330–6. https://doi.org/10.1038/ni904.
Sakaguchi S, Miyara M, Costantino CM, et al. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10(7):490–500. https://doi.org/10.1038/nri2785.
Xue L, Lu HQ, He J, et al. Expression of FOXP3 in esophageal squamous cell carcinoma relating to the clinical data. Dis Esophagus. 2010;23(4):340–6. https://doi.org/10.1111/j.1442-2050.2009.01013.x.
Wang G, Liu G, Liu Y, et al. FOXP3 expression in esophageal cancer cells is associated with poor prognosis in esophageal cancer. Hepatogastroenterology. 2012;59(119):2186–91. https://doi.org/10.5754/hge11961.
Li C, Yang W, Gai X, Zhang Y, et al. Foxp3 overexpression decreases sensitivity to chemotherapy in mouse Lewis lung cancer cells. Mol Med Rep. 2012;6(5):977–82. https://doi.org/10.3892/mmr.2012.1066.
Wang G, Sun Y, Ji C, et al. Correlation between the CD4+CD25high regulatory T cells and the outcome of chemotherapy in advanced esophageal carcinoma. Hepatogastroenterology. 2013;60(124):704–8. https://doi.org/10.5754/hge12953.
Xu T, Duan Q, Wang G, et al. CD4 + CD25high regulatory T cell numbers and FOXP3 mRNA expression in patients with advanced esophageal cancer before and after chemotherapy. Cell Biochem Biophys. 2011;61(2):389–92. https://doi.org/10.1007/s12013-011-9197-1.
Li C, Sun L, Jiang R, et al. Downregulation of FOXP3 inhibits cell proliferation and enhances chemosensitivity to cisplatin in human lung adenocarcinoma. Pathol Res Pract. 2017;213(10):1251–6. https://doi.org/10.1016/j.prp.2017.09.004.
Tanaka A, Nishikawa H, Noguchi S, et al. Tyrosine kinase inhibitor imatinib augments tumor immunity by depleting effector regulatory T cells. J Exp Med. 2020;217(2):e20191009. https://doi.org/10.1084/jem.20191009.
Isomoto K, Haratani K, Hayashi H, et al. Impact of EGFR-TKI Treatment on the Tumor Immune Microenvironment in EGFR Mutation-Positive Non-Small Cell Lung Cancer. Clin Cancer Res. 2020;26(8):2037–46. https://doi.org/10.1158/1078-0432.CCR-19-2027.
Sacco AG, Chen R, Worden FP, et al. Pembrolizumab plus cetuximab in patients with recurrent or metastatic head and neck squamous cell carcinoma: an open-label, multi-arm, non-randomised, multicentre, phase 2 trial. Lancet Oncol. 2021;22(6):883–92. https://doi.org/10.1016/S1470-2045(21)00136-4.
Chung CH, Li J, Steuer CE, et al. Phase II multi-institutional clinical trial result of concurrent cetuximab and nivolumab in recurrent and/or metastatic head and neck squamous cell carcinoma. Clin Cancer Res. 2022;28(11):2329–38. https://doi.org/10.1158/1078-0432.CCR-21-3849.
Ito M, Komai K, Mise-Omata S, et al. Brain regulatory T cells suppress astrogliosis and potentiate neurological recovery. Nature. 2019;565(7738):246–50. https://doi.org/10.1038/s41586-018-0824-5.
Jiang R, Tang J, Chen Y, et al. The long noncoding RNA lnc-EGFR stimulates T‑regulatory cells differentiation thus promoting hepatocellular carcinoma immune evasion. Nat Commun. 2017;8:15129. https://doi.org/10.1038/ncomms15129.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
SHW and YW designed the experiments. ZJZ and JFZ performed the experiments. ZHH and MY collected and analyzed the data. ZHH and MY drafted manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
S. Wu, Y. Wang, Z. Xiao, J. Zhang, Z. He and M. Ye declare that they have no competing interests.
Ethical standards
The current study was approved by the Animal Ethics Committee and was conducted in accordance with the relevant agreements with Renji Hospital. All procedures were performed in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of China. Consent for Publication: not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shenghong Wu and Yu Wang are the first co-authors and contributed equally to this work.
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Rights and permissions
About this article
Cite this article
Wu, S., Wang, Y., Xiao, Z. et al. FOXP3 expression in esophageal squamous cell carcinoma. Wien Klin Wochenschr (2023). https://doi.org/10.1007/s00508-023-02291-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00508-023-02291-4