Summary
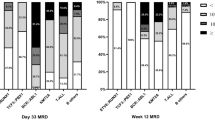
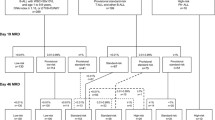
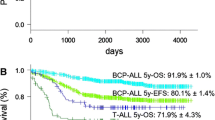
Since 1979 Austrian children and adolescents with acute lymphoblastic leukemia (ALL) have been treated according to protocols of the Berlin-Frankfurt-Münster (BFM) study group. The Associazione Italiana di Ematologia e Oncologia Pediatrica and BFM (AIEOP-BFM) ALL 2000 study was designed to prospectively study patient stratification into three risk groups using minimal residual disease (MRD) on two time points during the patient’s early disease course. The MRD levels were monitored by detection of clone-specific rearrangements of the immunoglobulin and T‑cell receptor genes applying a quantitative polymerase chain reaction-based technique. The 7‑year event-free survival (EFS) and overall survival rates for all 608 Austrian patients treated between June 1999 and December 2009 within the AIEOP-BFM 2000 study were 84 ± 2% and 91 ± 1%, respectively, with a median observation time of 6.58 years. Event-free survival for patients with precursor B‑cell and T‑cell ALL were 84 ± 2% (n = 521) and 84 ± 4% (n = 87; p = 0.460), respectively. The MRD assessment was feasible in 94% of the patients and allowed the definition of precursor B‑cell ALL patients with a low, intermediate or high risk of relapse even on top of clinically relevant subgroups. A similar finding with respect to MRD relevance in T‑ALL patients was not possible due to the small number of patients and events. Since this pivotal international AIEOP-BFM ALL 2000 trial, molecular response to treatment has been continuously used with additional refinements to stratify patients into different risk groups in all successive trials of the AIEOP-BFM ALL study group.





Similar content being viewed by others
References
Hunger SP, Mullighan CG. Acute lymphoblastic leukemia in children. N Engl J Med. 2015;373(16):1541–52.
Pui CH. Recent advances in childhood acute lymphoblastic leukemia. J Formos Med Assoc. 2004;103(2):85–95.
Pui CH. Precision medicine in acute lymphoblastic leukemia. Front Med. 2020;14(6):689–700.
Moricke A, Zimmermann M, Reiter A, Gadner H, Odenwald E, Harbott J, et al. Prognostic impact of age in children and adolescents with acute lymphoblastic leukemia: data from the trials ALL-BFM 86, 90, and 95. Klin Padiatr. 2005;217(6):310–20.
Moricke A, Zimmermann M, Reiter A, Henze G, Schrauder A, Gadner H, et al. Long-term results of five consecutive trials in childhood acute lymphoblastic leukemia performed by the ALL-BFM study group from 1981 to 2000. Leukemia. 2010;24(2):265–84.
Pieters R, de Groot-Kruseman H, Van der Velden V, Fiocco M, van den Berg H, de Bont E, et al. Successful therapy reduction and intensification for childhood acute lymphoblastic leukemia based on minimal residual disease monitoring: study ALL10 from the Dutch childhood oncology group. J Clin Oncol. 2016;34(22):2591–601.
Schmiegelow K, Forestier E, Hellebostad M, Heyman M, Kristinsson J, Soderhall S, et al. Long-term results of NOPHO ALL-92 and ALL-2000 studies of childhood acute lymphoblastic leukemia. Leukemia. 2010;24(2):345–54.
Biondi A, Valsecchi MG, Seriu T, D’Aniello E, Willemse MJ, Fasching K, et al. Molecular detection of minimal residual disease is a strong predictive factor of relapse in childhood B‑lineage acute lymphoblastic leukemia with medium risk features. A case control study of the International BFM study group. Leukemia. 2000;14(11):1939–43.
Conter V, Bartram CR, Valsecchi MG, Schrauder A, Panzer-Grumayer R, Moricke A, et al. Molecular response to treatment redefines all prognostic factors in children and adolescents with B‑cell precursor acute lymphoblastic leukemia: results in 3184 patients of the AIEOP-BFM ALL 2000 study. Blood. 2010;115(16):3206–14.
Coustan-Smith E, Sancho J, Hancock ML, Boyett JM, Behm FG, Raimondi SC, et al. Clinical importance of minimal residual disease in childhood acute lymphoblastic leukemia. Blood. 2000;96(8):2691–6.
Schrappe M, Valsecchi MG, Bartram CR, Schrauder A, Panzer-Grumayer R, Moricke A, et al. Late MRD response determines relapse risk overall and in subsets of childhood T‑cell ALL: results of the AIEOP-BFM-ALL 2000 study. Blood. 2011;118(8):2077–84.
van Dongen JJ, Seriu T, Panzer-Grumayer ER, Biondi A, Pongers-Willemse MJ, Corral L, et al. Prognostic value of minimal residual disease in acute lymphoblastic leukaemia in childhood. Lancet. 1998;352(9142):1731–8.
Willemse MJ, Seriu T, Hettinger K, d’Aniello E, Hop WC, Panzer-Grumayer ER, et al. Detection of minimal residual disease identifies differences in treatment response between T‑ALL and precursor B‑ALL. Blood. 2002;99(12):4386–93.
Zhou J, Goldwasser MA, Li A, Dahlberg SE, Neuberg D, Wang H, et al. Quantitative analysis of minimal residual disease predicts relapse in children with B‑lineage acute lymphoblastic leukemia in DFCI ALL consortium protocol 95-01. Blood. 2007;110(5):1607–11.
Dworzak MN, Froschl G, Printz D, Mann G, Potschger U, Muhlegger N, et al. Prognostic significance and modalities of flow cytometric minimal residual disease detection in childhood acute lymphoblastic leukemia. Blood. 2002;99(6):1952–8.
Pieters R, Schrappe M, De Lorenzo P, Hann I, De Rossi G, Felice M, et al. A treatment protocol for infants younger than 1 year with acute lymphoblastic leukaemia (Interfant-99): an observational study and a multicentre randomised trial. Lancet. 2007;370(9583):240–50.
Biondi A, Schrappe M, De Lorenzo P, Castor A, Lucchini G, Gandemer V, et al. Imatinib after induction for treatment of children and adolescents with Philadelphia-chromosome-positive acute lymphoblastic leukaemia (EsPhALL): a randomised, open-label, intergroup study. Lancet Oncol. 2012;13(9):936–45.
van der Does-van den Berg A, Bartram CR, Basso G, Benoit YC, Biondi A, Debatin KM, et al. Minimal requirements for the diagnosis, classification, and evaluation of the treatment of childhood acute lymphoblastic leukemia (ALL) in the “BFM family” cooperative group. Med Pediatr Oncol. 1992;20(6):497–505.
Bene MC, Castoldi G, Knapp W, Ludwig WD, Matutes E, Orfao A, et al. Proposals for the immunological classification of acute leukemias. European group for the immunological characterization of leukemias (EGIL). Leukemia. 1995;9(10):1783–6.
Attarbaschi A, Mann G, Konig M, Dworzak MN, Trebo MM, Muhlegger N, et al. Incidence and relevance of secondary chromosome abnormalities in childhood TEL/AML1+ acute lymphoblastic leukemia: an interphase FISH analysis. Leukemia. 2004;18(10):1611–6.
Reismuller B, Steiner M, Pichler H, Dworzak M, Urban C, Meister B, et al. High hyperdiploid acute lymphoblastic leukemia (ALL)-A 25-year population-based survey of the Austrian ALL-BFM (Berlin-Frankfurt-Munster) study group. Pediatr Blood Cancer. 2017; https://doi.org/10.1002/pbc.26327.
Flohr T, Schrauder A, Cazzaniga G, Panzer-Grumayer R, van der Velden V, Fischer S, et al. Minimal residual disease-directed risk stratification using real-time quantitative PCR analysis of immunoglobulin and T‑cell receptor gene rearrangements in the international multicenter trial AIEOP-BFM ALL 2000 for childhood acute lymphoblastic leukemia. Leukemia. 2008;22(4):771–82.
Attarbaschi A, Mann G, Zimmermann M, Bader P, Barisone E, Basso G, et al. Randomized post-induction and delayed intensification therapy in high-risk pediatric acute lymphoblastic leukemia: long-term results of the international AIEOP-BFM ALL 2000 trial. Leukemia. 2020;34(6):1694–700.
Locatelli F, Valsecchi MG, Moricke A, Zimmermann M, Gruhn B, Biondi A, et al. Protocol II vs protocol III given twice during reinduction therapy in children with medium-risk ALL. Blood. 2017;130(19):2146–9.
Schrappe M, Bleckmann K, Zimmermann M, Biondi A, Moricke A, Locatelli F, et al. Reduced-intensity delayed intensification in standard-risk pediatric acute lymphoblastic leukemia defined by undetectable minimal residual disease: results of an international randomized trial (AIEOP-BFM ALL 2000). J Clin Oncol. 2018;36(3):244–53.
Moricke A, Zimmermann M, Valsecchi MG, Stanulla M, Biondi A, Mann G, et al. Dexamethasone vs prednisone in induction treatment of pediatric ALL: results of the randomized trial AIEOP-BFM ALL 2000. Blood. 2016;127(17):2101–12.
Kalbfleisch JD, Prentice RL. The statisticalanalysis of failure time data. New York: JohnWiley and Sons; 1980, 163–188.
Hunger SP, Winick NJ, Sather HN, Carroll WL. Therapy of low-risk subsets of childhood acute lymphoblastic leukemia: when do we say enough? Pediatr Blood Cancer. 2005;45(7):876–80.
Bertaina A, Vinti L, Strocchio L, Gaspari S, Caruso R, Algeri M, et al. The combination of bortezomib with chemotherapy to treat relapsed/refractory acute lymphoblastic leukaemia of childhood. Br J Haematol. 2017;176(4):629–36.
von Stackelberg A, Locatelli F, Zugmaier G, Handgretinger R, Trippett TM, Rizzari C, et al. Phase I/phase II study of blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. J Clin Oncol. 2016;34(36):4381–9.
Gaynon PS, Desai AA, Bostrom BC, Hutchinson RJ, Lange BJ, Nachman JB, et al. Early response to therapy and outcome in childhood acute lymphoblastic leukemia: a review. Cancer. 1997;80(9):1717–26.
Larsen EC, Devidas M, Chen S, Salzer WL, Raetz EA, Loh ML, et al. Dexamethasone and high-dose methotrexate improve outcome for children and young adults with high-risk B‑acute lymphoblastic leukemia: a report from children’s oncology group study AALL0232. J Clin Oncol. 2016;34(20):2380–8.
Seibel NL, Steinherz PG, Sather HN, Nachman JB, Delaat C, Ettinger LJ, et al. Early postinduction intensification therapy improves survival for children and adolescents with high-risk acute lymphoblastic leukemia: a report from the children’s oncology group. Blood. 2008;111(5):2548–55.
Toft N, Birgens H, Abrahamsson J, Griskevicius L, Hallbook H, Heyman M, et al. Results of NOPHO ALL2008 treatment for patients aged 1–45 years with acute lymphoblastic leukemia. Leukemia. 2018;32(3):606–15.
Vora A, Goulden N, Mitchell C, Hancock J, Hough R, Rowntree C, et al. Augmented post-remission therapy for a minimal residual disease-defined high-risk subgroup of children and young people with clinical standard-risk and intermediate-risk acute lymphoblastic leukaemia (UKALL 2003): a randomised controlled trial. Lancet Oncol. 2014;15(8):809–18.
Arico M, Conter V, Valsecchi MG, Rizzari C, Boccalatte MF, Barisone E, et al. Treatment reduction in highly selected standard-risk childhood acute lymphoblastic leukemia. The AIEOP ALL-9501 study. Haematologica. 2005;90(9):1186–91.
Moricke A, Reiter A, Zimmermann M, Gadner H, Stanulla M, Dordelmann M, et al. Risk-adjusted therapy of acute lymphoblastic leukemia can decrease treatment burden and improve survival: treatment results of 2169 unselected pediatric and adolescent patients enrolled in the trial ALL-BFM 95. Blood. 2008;111(9):4477–89.
Schrappe M, Reiter A, Ludwig WD, Harbott J, Zimmermann M, Hiddemann W, et al. Improved outcome in childhood acute lymphoblastic leukemia despite reduced use of anthracyclines and cranial radiotherapy: results of trial ALL-BFM 90. German-Austrian-Swiss ALL-BFM study group. Blood. 2000;95(11):3310–22.
Borowitz MJ, Devidas M, Hunger SP, Bowman WP, Carroll AJ, Carroll WL, et al. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia and its relationship to other prognostic factors: a children’s oncology group study. Blood. 2008;111(12):5477–85.
Cave H, van der Werff ten Bosch J, Suciu S, Guidal C, Waterkeyn C, Otten J, et al. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia. European organization for research and treatment of cancer—childhood leukemia cooperative group. N Engl J Med. 1998;339(9):591–8.
Basso G, Veltroni M, Valsecchi MG, Dworzak MN, Ratei R, Silvestri D, et al. Risk of relapse of childhood acute lymphoblastic leukemia is predicted by flow cytometric measurement of residual disease on day 15 bone marrow. J Clin Oncol. 2009;27(31):5168–74.
Maurer-Granofszky M, Schumich A, Buldini B, Gaipa G, Kappelmayer J, Mejstrikova E, et al. An extensive quality control and quality assurance (QC/QA) program significantly improves inter-laboratory concordance rates of flow-cytometric minimal residual disease assessment in acute lymphoblastic leukemia: an I‑BFM-FLOW-network report. Cancers (Basel). 2021;13(23):6148. https://doi.org/10.3390/cancers13236148.
Testi AM, Attarbaschi A, Valsecchi MG, Moricke A, Cario G, Niggli F, et al. Outcome of adolescent patients with acute lymphoblastic leukaemia aged 10–14 years as compared with those aged 15–17 years: long-term results of 1094 patients of the AIEOP-BFM ALL 2000 study. Eur J Cancer. 2019;122:61–71.
Acknowledgements
We thank all participating institutions, nurses, psychosocial workers and physicians for their support of the study.
Funding
This work was supported by the St. Anna Kinderkrebsforschung (St. Anna Children’s Cancer Research Institute), Austria.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
L. Ronceray, M. Dworzak, K. Dieckmann, G. Ebetsberger-Dachs, E. Glogova, O.A. Haas, N. Jones, K. Nebral, R. Moser, T. Lion, B. Meister, R. Panzer-Grümayer, S. Strehl, C. Peters, U. Pötschger, C. Urban, G. Mann and A. Attarbaschi declare that they have no competing interests.
Ethical standards
Institutional review board statement: the AIEOP-BFM ALL 2000 trial in Austria was approved by the institutional ethics committees of the coordinating center in Vienna as well as of all other participating institutions. Informed consent statement: informed consent was obtained from all patients enrolled in the AIEOP-BFM ALL 2000 trial in Austria.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors Georg Mann and Andishe Attarbaschi share senior authorship.
This paper is dedicated to our friend and mentor Helmut Gadner, the former Head of the St. Anna Children Hospital and founder of the Children’s Cancer Research Institute, Vienna, Austria, who chaired the Austrian ALL-BFM Study Group for 30 years and was not only instrumental in conceiving and conducting the Austrian ALL therapy studies, but also to get our clinical and diagnostic achievements internationally recognized.
Rights and permissions
About this article
Cite this article
Ronceray, L., Dworzak, M., Dieckmann, K. et al. Prospective use of molecular minimal residual disease for risk stratification in children and adolescents with acute lymphoblastic leukemia. Wien Klin Wochenschr (2023). https://doi.org/10.1007/s00508-023-02249-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00508-023-02249-6