Summary
Background
Merkel cell carcinomas (MCC) are very aggressive tumors of the sun-exposed skin with a high potential to metastasize. Little is known about the genesis of MCC and very few prognostic markers have been detected so far. The Wnt pathway protein β-catenin and the cell cycle protein cyclin D1 are two promotors of tumor growth and are expressed in a variety of malignant neoplasms such as lymphomas, thyroid, breast cancer, and many others.
Patients and Methods
Tissue samples of 27 patients with MCC were immunohistochemically stained for β-catenin and cyclin D1 and correlated with overall survival of patients. In addition, western blot analysis was carried out in the two MCC cell lines MCC-13 and MCC-26.
Results
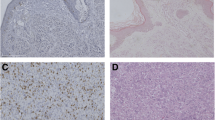
β-catenin showed a cytoplasmatic expression of 10–30 % in 11 samples and an expression lower than 10 % in eight samples. Nuclear staining was visible in two samples. None of the 27 samples expressed cyclin D1.
Conclusion
Neither cyclin D1 nor β-catenin was expressed in a statistically significant manner, concluding that the development of MCCs is independent of β-catenin and cyclin D1 expression and these proteins are not suitable as prognostic markers. We could describe the expression pattern of cyclin D1 for the first time.
Zusammenfassung
Hintergrund
Merkelzellkarzinome (MCC) sind äußerst aggressive Tumore der sonnenexponierten Haut mit einem hohen Metastasierungspotential. Über die Entstehung der MCC ist wenig bekannt und es wurden bisher nur wenige prognostische Marker beschrieben. Das Wnt pathway Protein β-catenin und der Zellzyklusaktivator Cyclin D1 sind zwei Promoter des Tumorwachstums und können in einer Vielzahl maligner Neubildungen wie Lymphomen, Schilddrüsen- und Mammakarzinomen nachgewiesen werden.
Patienten und Methoden
Die Gewebeproben von 27 Patienten mit MCC wurden immunhistochemisch mit Antikörpern für β-catenin und Cyclin D1 gefärbt und mit dem Gesamtüberleben der Patienten korreliert. Zusätzlich wurden Western blot Analysen in zwei MCC Zelllinien, MCC-13 und MCC-26, durchgeführt.
Ergebnisse
β-catenin zeigte eine zytoplasmatische Expression von 10–30 % in 11 Patientenproben und eine geringere Expression (< 10 %) in acht Proben. Eine Kernfärbung konnte nur in zwei Proben beobachtet werden. Keine der 27 Patientenproben zeigte eine Expression von Cyclin D1.
Schlussfolgerung
Weder Cyclin D1 noch β-catenin zeigten eine statistisch relevante Expression, woraus man schlussfolgern kann, dass die Entstehung der MCC unabhängig von einer β-catenin und Cyclin D1 Expression stattfindet. Weiters können diese beiden Proteine nicht als prognostische Marker herangezogen werden. Das Expressionsmuster von Cyclin D1 in MCC wurde in dieser Arbeit erstmals beschrieben.


Similar content being viewed by others
References
Lassacher A, Heitzer E, Kerl H, et al. P15ARF hypermethylation is common but INK4a-ARF locus or p53 mutations are rare in Merkel cell carcinoma. J Invest Dermatol. 2008;128:1788–96.
Becker JC, Schrama D, Houben R. Merkel cell carcinoma. Cell Mol Life Sci. 2008;66:1–8.
Gedlicka C, Hager G, Weissenböck M, et al. 1,25 (OH)2 Vitamin D3 induces elevated expression of the cell cycle inhibitor p18 in a squamous cell carcinoma cell line of the head and neck. J Oral Pathol Med. 2006;35:472–8.
Weller K, Vetter-Kauzcok C, Kähler K, et al. Guideline implementation in Merkel cell carcinoma: an example of a rare disease. Dtsch Aerztebl. 2008;103:2791–6.
Lill C, Schneider S, Pammer J, et al. Significant correlation of peptidylprolyl isomerase overexpression in Merkel cell carcinoma with overall survival of patients. Head Neck. 2011 Sep;33(9):1294–300.
Woo K, Choi Y, Jung HS, et al. Merkel cell carcinoma: our experience with seven patients in Korea and a literature review. J Plast Reconstr Aesthet Surg. 2010 Dec;63(12):2064–70.
Zampetti A, Feliciani C, Massi G, Tulli A. Updated review of the pathogenesis and management of Merkel cell carcinoma. J Cutan Med Surg. 2010;14:51–61.
Zhou CX, Gao Y. Aberrant expression of beta-catenin, PIN1 and cyclin D1 in salivary adenoid cystic carcinoma: relation to tumor proliferation and metastasis. Oncol Rep. 2006;16(3):505–11.
Guo Q, Wu M, Lian P, et al. Synergistic effect of indomethacin and NGX6 on proliferation and invasion by human colorectal cancer cells through modulation of the Wnt/beta-catenin signaling pathway. Mol Cell Biochem. 2009;330(1-2):71–81.
Khramtsov AI, Khramtsova GF, Tretiakova M, et al. Wnt/β-catenin pathway activation is enriched in basal-like breast cancers and predicts poor outcome. Am J Pathol. 2010;176(6):2911–20.
Hager G, Formanek M, Gedlicka C, et al. 1,25(OH)2Vitamin D3 induces elevated expression of the cell cycle-regulating genes p21 and p27 in squamous carcinoma cell lines of the head and neck. Acta Otolarygol. 2001;121:103–9.
Bassiouny AE, Nosseir MM, Zoheiry MK, et al. Differential expression of cell cycle regulators in HCV-infection and related hepatocellular carcinoma. World J Hepatol. 2010;2(1):32–41.
Cao X, Fan L, Fang C, et al. The expression of SOX11, cyclin D1, cyclin D2, and cyclin D3 in B-cell lymphocytic proliferative diseases. Med Oncol. 2012;29(2):1190–6.
Nakashima M, Meirmanov S, Naruke Y, et al. Cyclin D1 overexpression in thyroid tumours from a radio-contaminated area and its correlation with Pin1 and aberrant β-catenin expression. J Pathol. 2004;202:446–55.
Peng H, Zhong XY, Liu KP, Li SM. Expression and significance of adenomatous polyposis coli, beta-catenin, E-cadherin and cyclin D1 in esophageal squamous cell carcinoma assessed by tissue microarray. Ai Zheng. 2009;28(1):38–41.
Yiengpruksawan A, Coit DG, Thaler HT, et al. Merkel cell carcinoma. Prognosis and management. Arch Surg. 1991;126:1514–9.
Beasley MB, Lantuejoul S, Abbondanzo S, et al. The p16/cyclin D1/Rb pathway in neuroendocrine tumors of the lung. Hum Pathol. 2003;34(2):136–42.
Sur M, Al Ardati H, Ross C, et al. TdT expression in Merkel cell carcinoma: potential diagnostic pitfall with blastic haematological malignancies and expanded immunohistochemical analysis. Mod Pathol. 2007;20:1113–20.
Brunner M, Thurnher D, Pammer J, et al. Expression of hedgehog signaling molecules in Merkel cell carcinoma. Head Neck. 2009;32:333–40.
Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54.
Brunner M, Thurnher D, Pammer J, et al. Expression of VEGF-A/C, VEGF-R2, PDGF-alpha/beta, c-kit, EGFR, Her-2/Neu, Mcl-1 and Bmi-1 in Merkel cell carcinoma. Mod Pathol. 2008;21:876–84.
Feinmesser M, Halpern M, Kaganovsky E, et al. C-kit expression in primary and metastatic Merkel cell carcinoma. Am J Dermatopathol. 2004;26:458–62.
Llombart B, Monteagudo C, Lopez-Guerrero JA, et al. Clinicopathological and immunohistochemical analysis of 20 cases of Merkel cell carcinoma in search of prognostic markers. Histopathology. 2005;46:622–34.
Strong S, Shalders K, Carr R, Snead DR. KIT receptor (CD 117) expression in Merkel cell carcinoma. Br J Dermatol. 2004;150:384–5.
Feinmesser M, Halpern M, Fenig E, et al. Expression of the apoptosis-related oncogenes bcl-2, bax, and p53 in Merkel cell carcinoma: can they predict treatment response and clinical outcome? Hum Pathol. 1999;30:1367–72.
Kennedy MM, Blessing K, King G, Kerr KM. Expression of bcl-2 and p53 in Merkel cell carcinoma. An immunohistochemical study. Am J Dermatopathol. 1996;18:273–7.
Houben R, Shuda M, Weinkam R, et al. Merkel cell polyomavirus-infected Merkel cell carcinoma cells require expression of viral T antigens. J Virol. 2010;84(14):7064–72.
Declercq J, Van Dyck F, Van Damme B, Van de Ven WJ. Upregulation of Igf and Wnt signalling associated genes in pleomorphic adenomas of the salivary glands in PLAG1 transgenic mice. Int J Oncol. 2008;32(5):1041–7.
Lill C, Schneider S, Item CB, et al. P53 mutation is a rare event in Merkel cell carcinoma of the head and neck. Eur Arch Otorhinolaryngol. 2011;268(11):1639–46.
Nordkvist A, Roijer E, Bang G, et al. Expression and mutation patterns of p53 in benign and malignant salivary gland tumors. Int J Oncol. 2000;16(3):477–83.
Freitas L, Araujo V, Martins M, et al. Biomarker analysis in carcinoma ex pleomorphic adenoma at an early phase of carcinomatous transformation. Int J Surg Path. 2005;13(4):337–42.
Girschik J, Fritschi L, Threlfall T, et al. Deaths from non-melanoma skin cancer in western Australia. Cancer Causes Control. 2008;19:879–85.
Lamb P, Crawford L. Characterization of the human p53 gene. Mol Cell Biol. 1986;6(5):1379–85.
Wodarz A, Nusse R. Mechanisms of Wnt signalling in development. Annu Rev Cell Dev Biol. 1998;14:59–88.
Williams JM, SH Oh, Jorgensen M, et al. The role of the Wnt family of secreted proteins in rat oval “stem” cell-based liver regeneration. Am J Pathol. 2010;176(6):2732–42.
Goan Y, Chang H, Hsu H, et al. Risk of p53 gene mutation in esophageal squamous cell carinoma and habit of betel quid chewing in Taiwanese. Cancer Sci. 2005;96(11):758–65.
Liu S, Daa T, Kashima K, et al. The Wnt-signaling pathway is not implicated in tumorigenesis of Merkel cell carcinoma. J Cutan Pathol. 2007;34(2):22–6.
Conflict of interest
The authors state no conflict of interest or any financial disclosure.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lill, C., Schneider, S., Ghanim, B. et al. Expression of β-catenin and cyclin D1 in Merkel cell carcinomas of the head and neck. Wien Klin Wochenschr 125, 501–507 (2013). https://doi.org/10.1007/s00508-013-0406-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00508-013-0406-3