Abstract
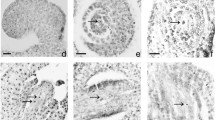
Information on the development of the male reproductive structures in willow will help advance our understanding of its reproductive behavior and contribute to our ability to work towards its improvement. Willow also offers the opportunity to study male sterility, a subject matter which is not typically dealt with in woody plants. As compared to the three willow species examined (Salix eriocephala, S. exigua, and S. purpurea), pollen development in S. discolor ‘S365’ showed several abnormalities starting with the delay in meiosis. This lasted for about 10 days and meiosis eventually occurred as manifested by the formation of microspores. However, most of the resulting microspores collapsed, while only a few developed into pollen grains. The large number of undeveloped and disintegrated microspores appeared to make the few pollen grains sticky, preventing them from being dispersed. Histochemical analysis showed that meiosis in most species of willow was associated with the presence of large amounts of insoluble polysaccharides in the anther wall layers, but only very few of these were observed in S. discolor. Also, a 32-kDa protein which is the most abundant protein in the reproductive structures of willow, was absent in S. discolor ‘S365’. Proteomic analysis showed that this is similar to the storage proteins in Populus x canadensis and P. deltoides. Therefore, male sterility in S. discolor may be due to some genetic defects affecting the accumulation of essential reserves in its reproductive structures. The mechanism behind this is unknown, but this study has established the nature of sterility in S. discolor ‘S365’.





Similar content being viewed by others
References
Alstrom-Rapaport C, Lascoux M, Wang YC, Roberts G, Tuskan GA (1998) Identification of a RAPD marker linked to sex determination in the basket willow (Salix viminalis L.). J Hered 89:44–49
Aravanopoulos FA (2000) Absence of association between heterozygosity and biomass production in Salix exigua Nutt. Theor Appl Genet 100:1203–1208
Banga SS, Laban KS, Banga SK (1984) Male sterility in Indiana mustard (Brassica juncea (L.) Coss.) – a biochemical characterization. Theor Appl Genet 67:515–519
Barker JHA, Pahlich A, Trybush S, Edwards KJ, Karp A (2003) Microsatellite markers for diverse Salix species. Mol Ecol Notes 3:4–6
Beardmore T, Wetzel S, Burgess D, Charest P J (1996) Characterization of seed storage proteins in Populus and their homology with Populus vegetative storage proteins. Tree Physiol 16: 833–840
Bhadula SK, Sawhney VK (1989) Amylolytic activity and carbohydrate levels during the stamen ontogeny of a male fertile and a “gibberellin-sensitive” male sterile mutant tomato (Lycopersicon esculentum). J Exp Bot 40:789–794
Borjesson P (1999) Environmental effects of energy crop cultivation in Sweden-I: identification and quantification. Biomass Bioenergy 16:137–154
Chaubal R, Zanella C, Trimnell MR, Fox TW, Albertsen MC, Bedinger P (2000) Two male-sterile mutants of Zea mays (Poaceae) with an extra cell division in the anther wall. Am J Bot 87:1193–1201
Clausen S, Apel K (1991) Seasonal changes in the concentration of the major storage protein and its mRNA in xylem ray cells of poplar trees. Plant Mol biol 17:669–678
Clément C, Audran JC (1995) Anther wall layers control pollen sugar nutrition in Lilium. Protoplasma 187:172–181
Clément C, Audran JC (1999) In: Clément C, Pacini E, Audran JC (eds) Anther and Pollen. From biology to biotechnology. Springer, Heidelberg Berlin Newyork, pp 69–90
Clément C, Chavant L, Burrus M, Audran JC (1994) Anther starch variations in Lilium during pollen development. Sex Plant Reprod 7:347–356
Clément C, Burrus M, Audran JC (1996) Floral organ growth and carbohydrate content during pollen development in Lilium. Am J Bot 83:459–469
Coleman GD, Chen THH (1993) Sequence of a poplar bark storage protein gene. Plant Physiol 102:1347–3–1348
Coleman GD, Chen THH, Fuchigami L (1992) cDNA cloning of poplar bark storage protein and control of its expression by photoperiod. Plant Physiol 98:687–693
Daniell H (2002) Molecular strategies for gene containment in transgenic crops. Nat Biotechnol 20:581–586
Datta R, Chamusco KC, Chourey PS (2002) Starch biosynthesis during pollen maturation is associated with altered patterns of gene expression in maize. Plant Physiol 130:1645–1656
Eschrich W, Currier HB (1964) Identification of callose by its diachrome and fluorochrome reactions. Stain Technol 39:303–307
Feder N, O’Brien TP (1968) Plant microtechnique; some principles and new methods. Am J Bot 55:123–142
Fernando DD, Owens JN, Yu X, Ekramoddoullah AKM (2001) RNA and protein synthesis during in vitro pollen germination and tube elongation in Pinus monticola and other conifers. Sex Plant Reprod 13:259–264
Fielding JM (1960) Branching and flowering characteristics of Monterey pine. Forestry and timber bureau bulletin 37. Commonwealth Government Printer, Canberra, Australia
Goetz M, Godt DE, Guivarch A, Kahmann U, Chriqui D, Roitsch T (2001) Induction of male sterility in plants by metabolic engineering of the carbohydrate supply. Proc Natl Acad Sci USA 98:6522–6527
Golan-Goldhirsh A, Peri I, Birk Y (1998) Inflorescence bud proteins of Pistacia vera. Trees 12:415–419
Gorman SW, Banasiak D, Fairley C, Cormick S (1996) A 610 kb YAC clone harbors 7 cM of tomato (Lycopersicon esculentum) DNA that includes the male sterile 14 gene and a hotspot for recombination. Mol Gen Genet 251:52–59
Guerineau F, Sorensen A, Fenby N, Scott RJ (2003) Temperature sensitive diphtheria toxin confers conditional male-sterility in Arabidopsis thaliana. Plant Biotechnol J 1:33–42
Gunter L E, Roberts G T, Lee K, Larimer W, Tuskan A (2003) The development of two flanking SCAR markers linked to a sex determination locus in Salix viminalis L. J Hered 94:185–189
Hallgren P, Ikonen A, Hjalten J, Roininen H (2003) Inheritance patterns of phenolics in F1, F2, and back-cross hybrids of willows: implications for herbivore responses to hybrid plants. J Chem Ecol 29:1143–1158
Hanley S, Barker JHA, Van Ooijen JW, Aldam C, Harris SL, Ahman I, Larsson S, Karp A (2002) A genetic linkage map of willow (Salix viminalis) based on AFLP and microsatellite markers. Theor Appl Genet 105:1087–1096
Herman E, Larkins B (1999) Protein storage bodies and vacuoles. Plant Cell 11:601–613
Hunter T, Peacock L, Hunter H, Brain P (2002) Effect of plantation design on stem-infesting form of rust in willow biomass coppice. For Pathol 32:87–97
Jain SM, Minocha SC (2000) Molecular biology of woody plant. Vol. I. Kluwer Scientific Publisher, Dordrecht, The Netherlands, pp 135–153
Jean D, Lapointe L (2001) Limited carbohydrate availability as a potential cause of fruit abortion in Rubus chamaemorus. Physiol Plant 112:379–387
Kopp RF (2000) Genetic improvement of Salix using traditional breeding and AFLP fingerpring. PhD Dissertation. State University of New York College of Environmental Science and Forestry, Syracuse, NY.
Kopp RF, Smart LB, Maynard AC, Tuskan TA, Abrahamson LP (2002) Predicting within-family variability in juvenile height growth of Salix based upon similarity among parental AFLP fingerprints. Theor Appl Genet 105:106–112
Larsson S (1998) Genetic improvement of willow for short rotation coppice. Biomass Bioenergy 15:23–26
Lawrence DK, Mayne RG (1991) Plant growth regulators and photosynthesis. In: Baker NR, Percival MP (eds) Herbicides. Elsevier Science, New York, pp 299–335
Ledig FT, Linzer DIH (1978) Fuel crop breeding. Chemtech 8:18–27
Levings CS III (1993) Thoughts on cytoplasmic male sterility in cms-T maize. Plant Cell 5:1285–1290
Luna V, Lorenzo E, Reinoso H, Tordable MC, Abdala G, Pharis RP, Bottini R (1990) Dormancy in peach (Prunus persica L.) flower buds. I. Floral morphogenesis and endogenous gibberellins at the end of the dormancy period. Plant Physiol 93:20–25
McIlveen-Wright DR, Williams BC, McMullan JT (2001) A re-appraisal of wood-fired combustion. Bioresour Technol 76:183–190
Nienow S, McNamara KT, Gillespie AR (2000) Assessing plantation biomass for co-firing with coal in northern Indiana: a linear programming approach. Biomass Bioenergy 18:25–135
O’Farrell PH (1975) High resolution two-dimensional electrophoresis of proteins. J Biol Chem 250:4007–4021
Pacini E, Bellani LM, Lozzi R (1986) Pollen, tapetum and anther development in two cultivars of sweet cherry (Prunus avium). Phytomorphotogy 36:197–210
Perttu KL (1999) Environmental and hygienic aspects of willow coppice in Sweden. Biomass Bioenergy 16:291–297
Reinoso H, Luna V, Pharis RP, Bottini R (2002) Dormancy in peach (Prunus persica) flower buds. V. Anatomy of bud development in relation to phonological stage. Can J Bot 80:656–663
Saini HS (1997) Effects of water stress on male gametophyte development in plants. Sex Plant Reprod 10:67–73
Sawhney VK (1992) Floral mutants in tomato: development, physiology, and evolutionary implications. Can J Bot 70:707–710
Shykoff J, Kolokotronis SO, Collin C, López-Villavicencio (2003) Effects of male sterility on reproductive traits in gynodioecious plants: a meta-analysis. Oecologia 135:1–9
Smith MB, Palmer RG, Horner HT (2002) Microscopy of a cytoplasmic male-sterile soybean from an interspecific cross between Glycine max and G. soja (leguminosae). Am J Bot 89:417–426
Strauss SH, Rottmann WH, Brunner AM, Sheppard LA (1995) Genetic engineering of reproductive sterility in forest trees. Mol Breeding 1:5–26
Wetzel S, Greenwood JS (1991) The 32-kilodalton vegetative storage protein of Salix microstachya turz. Plant Physiol 97:771–777
Wilson E, Chapman PJ, McDonld A (2001) Merging nitrogen management and renewable energy needs. Sci World J 22:1 Suppl 2:745–749
Worrall D, Hird DL, Hodge R, Paul W, Draper J, Scott R (1992) Premature dissolution of the microsporocyte callose wall causes male sterility in transgenic tobacco. Plant Cell 4:759–771
Acknowledgements
Funding for this project was through the USDA McIntire Stennis Research Program. The assistance of Derek Smith, UVIC-Genome BC Proteomic Centre, in processing the protein sample for mass spectrometry is acknowledged.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zhang, S., Fernando, D.D. Structural, histochemical, and protein analysis of male reproductive development in willow. Sex Plant Reprod 18, 37–46 (2005). https://doi.org/10.1007/s00497-005-0249-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00497-005-0249-9