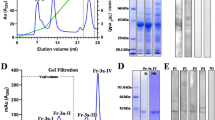
Abstract
Platanus acerifolia (Aiton) Willdenow is a plane tree, widely grown as an ornamental tree in many cities of the United States and Western Europe, which has become an important source of airborne allergens in our cities. The aim of the present study is to immunolocalize the major allergens in the pollen grain and to examine their potential function in the fertilization process. Observations were made in mature and hydrated, activated pollen of P. acerifolia for 5, 15, 30 min and 2 h in the germination medium. Specimens were fixed using freezing protocols for transmission electron microscopy (TEM). For immunogold labelling, cryosections and resin-embedded ultrathin sections were incubated using rabbit antisera against the purified pollen allergens Pla a 1 and Pla a 2. Elution of P. acerifolia allergens took place after 5 min of pollen incubation in buffered medium. Intense labelling of Pla a 1 and Pla a 2 was detected after pollen exudates were released. In pollen grains, Pla a 1 was predominantly localized in concentric cisternae of the endoplasmic reticulum (ER), situated between the vegetative nucleus and the generative cell, and was released from pollen grains 5 min after hydration; cytoplasmic localization decreased 15 min after hydration. In pollen grains, glycoprotein Pla a 2 was abundant in association with Golgi cisternae and vesicles situated in the apertural periphery of the mature pollen grains. Pla a 2 proteins were also detected in ER and in the generative cell wall. Immunolabelling of Pla a 2 decreased 5 min after pollen hydration but was again intense after 15–30 min in germination medium, presumably as a consequence of renewed expression and glycosylation of this protein. Pla a 1 belongs to a new class of allergens related to proteinaceous invertase and pectin methyl esterase inhibitors (PII, PMEI) which could be involved in membrane protection and pectin de-esterification control during pollen hydration. Pla a 2 has an exopolygalacturonase (PG) enzymatic activity consistent with pollen-stigma adhesion mechanisms or compatibility systems. Moreover, the expression of Pla a 2 found 15–30 min after hydration might contribute to pollen-tube growth and the modification of transmitting tissue cell walls. The abundant production and elution of Pla a 1 and Pla a 2 proteins may alter the environment in which pollen tube elongation occurs, thus promoting a potential crosstalk between the pollen and the gynoecium.








Similar content being viewed by others
References
Aceituno E, Del Pozo V, Minués A, Arrieta I, Cortesano B, Cardaba B, Gallardo S, Rojo M, Palomino P, Lahoz C (2000) Molecular cloning of major allergen from Cupressus arizonica pollen: Cup a 1. Clin Exp Allergy 30:1750–1758
Athanasiou A, Khosravi D, Tamari F, Shore JS (2003) Characterization and localization of short-specific polygalacturonase in distylous Turnera subulata (Turneraceae). Am J Bot 90:675–682
Brewbaker J, Kwack B (1964) The calcium ion and substances influencing pollen growth. In Linskens HF (ed) Pollen physiology and fertilization. Elsevier, Amsterdam, pp 145–151
Asturias JA, Bartolome B, Ojeda I, Malet A, Martinez A (2002) Purification and characterization of Pla a 1, a major allergen from Platanus acerifolia pollen. Allergy 57:221–227
Asturias JA, Ibarrola I, Eraso E, Arilla MC, Martínez A (2003) The major Platanus acerifolia pollen allergen Pla a 1 has sequence homology to invertase inhibitors. Clin Exp Allergy 33:978–985
Cadot P, Díaz JF, Proost P, van Damme J, Engelborghs Y, Stevens EAM, Ceuppens JL (2000) Purification and characterization of an 18-kd allergen of birch (Betula verrucosa) pollen: identification as a cyclophilin. J Allergy Clin Immunol 105:286–291
Casas C, Márquez J, Suárez-Cervera M, Seoane-Camba JA (1996) Immunocytochemical localization of allergenic proteins in Parietaria judaica L. (Urticaceae). Eur J Cell Biol 70:179–188
Castells T, Arcalís E, Moreno-Grau S, Bayo J, Elvira-Rendueles B, Belchí J, Seoane-Camba JA, Suárez-Cervera M (2002) Immunocytochemical localization of allergenic proteins from mature to activated Zygophyllum fabagoL. (Zygophyllaceae) pollen grains. Eur J Cell Biol 81:107–115
Castells T, Seoane-Camba JA, Suárez-Cervera M (2003) Intine wall modifications during germination of Zygophyllum fabago (Zygophyllaceae) pollen grains. Can J Bot 81:1267–1277
Clément C, Audran JC (1999) Anther carbohydrates during in vivo and in vitro pollen development. In: Clément C, Pacini E, Audran JC (eds) Anther and Pollen. From Biology to Biotechnology. Springer, Berlin Heidelberg New York, pp 69–90
Clément C, Burrus M, Audran JC (1996) Floral organ growth and carbohydrate content during pollen development in Lilium. Am J Bot 83:459–469
Dearnaley JDW, Daggard GA (2001) Expression of a polygalacturonase enzyme in germinating pollen of Brassica napus. Sex Plant Reprod 13:265–271
De Veau EJI, Gross KC, Huber DJ, Watada AE (1993) Degradation and solubilization of pectin by β-galactosidases purified from avocado mesocarp. Physiol Plant 87:279–285
Dorion S, Lalonde S, Saini HS (1996) Induction of male sterility in wheat by meiotic stage water deficit is preceded by a decline in invertase activity and changes in carbohydrate metabolism in anthers. Plant Physiol 111:137–145
Dubald M, Barakate A, Mandaron P, Mache R (1993) The ubiquitous presence of exopolygalacturonase in maize suggests a fundamental cellular function of this enzyme. Plant J 4:781–791
Geitmann A (1999) The reological properties of the pollen tube cell wall. In Cresti M, Cai G, Moscatelli A (eds) Fertilization in higher plants. Springer, Berlin Heidelberg New York, pp 283–297
Goldberg R, Morvan C, Jauneau A, Jarvis MC (1996) Methyl-esterification, de-esterification and gelation of pectins in the primary cell wall. In Visser J, Voragen AGJ (eds) Pectins and Pectinases. Elsevier, Amsterdam, pp 151–172
Grenier S, Köster U, Katja L, Rosenkranz H, Vogel R, Rausch T (2000) Plant invertase inhibitors: expression in cell culture and during plant development. Aust J Plant Physiol 27:807–814
Grote M, Vrtala S, Niederberger V, Wiermann R, Valenta R, Reichlt R (2001) Release of allergen-bearing cytoplasm from hydrated pollen: a mechanism common to a variety of grass (Poaceae) species revealed by electron microscopy. J Allergy Clin Immunol 108:109–115
Heslop-Harrison J (1987) Pollen germination and pollen tube growth. Int Rev Cytol 107:1–78
Hoekstra FA, van Roekel T (1988) Desiccation tolerance of Papaver dubium L. pollen during its development in the anther. Plant Physiol 88:626–632
Hoekstra FA, Crowe JH, van Roekel T, Vermeer E (1992) Do phospholipids and sucrose determine membrane phase transitions in dehydrating pollen species? Plant Cell Environ 15:601–606
Huecas S, Villalba M, Rodríguez R (2001) Ole e 9, a major olive pollen allergen is a 1,3-β-glucanase. J Biol Chem 276:27959–27066
Ibarrola I, Arilla MC, Martínez A, Asturias JA (2004) Identification of a polygalacturonase as a major allergen (Pla a 2) from Platanus acerifolia pollen. J Allergy Clin Immunol 113:1185–1191
Jauh GY, Lord EM (1996) Localization of pectins and arabinogalactan-proteins in lily (Lilium longiflorum L.) pollen tube and style, and their possible roles in pollination. Planta 199:251–261
Jian L, Yang S-L, Xie L-F, Puah CS, Zhang X-Q, Yang W-C, Sundaresan V, Ye D (2005) VANGUARD1 encodes a pectin methylesterase that enhances pollen tube growth in the Arabidopsis style and transmitting tract. Plant Cell 17:584–596
Khosravi D, Joulaie R, Shore JS (2003) Immunocytochemical distribution of polygalacturonase and pectins in styles of distylous and homostylous Turneraceae. Sex Plant Reprod 16:179–190
Knox RB, Heslop-Harrison J (1969) Cytochemical localization of enzymes in the wall of the pollen grain. Nature 223:92–94
Knox RB, Taylor PE, Ladiges P, Nelson G, Suphioglu C (1998) Pollen allergens: molecular and immunological analyses and implications for systematics. In: Owens SJ, Rudall PJ (eds) Reproductive biology. Royal Botanic Gardens, Kew, pp 449–463
Lennon KA, Lord EM (2000) In vivo pollen tube cell of Arabidopsis thaliana. Tube cell cytoplasm and wall. Protoplasma 214:45–56
Lennon KA, Roy S, Hepler PK, Lord EM (1998) The structure of the transmitting tissue of Arabidopsis thaliana L. and the path of pollen tube growth. Sex Plant Reprod 11:49–59
Li YQ, Chen F, Linskens HF, Cresti M (1994) Distribution of unesterified and esterified pectins in cell walls of pollen tubes of flowering plants. Sex Plant Reprod 7:145–152
Liou W, Geuze HJ, Slot JW (1996) Improving structural integrity of cryosections for immunogold labelling. Histochem Cell Biol 106:41–58
Lord EM, Russell SD (2002) The mechanisms of pollination and fertilization in plants. Annu Rev Cell Dev Biol 18:81–105.
Marchler-Bauer A, Bryant SH (2004) CD-Search: protein domain annotations on the fly. Nucleic Acids Res 32: W327–331
Marchler-Bauer A, Anderson JB, Cherukuri PF, DeWeese-Scott C, Geer LY, Gwadz M, He S, Hurwitz DI, Jackson JD, Ke Z, Lanczycki CJ, Liebert CA, Liu C, Lu F, Marchler GH, Mullokandov M, Shoemaker BA, Simonyan V, Song JS, Thiessen PA, Yamashita RA, Yin JJ, Zhang D, Bryant SH (2005) CDD: a Conserved Domain Database for protein classification. Nucleic Acids Res 33: D192–196
Markovič O, Janečedk S (2004) Pectin methylesterases: sequence-structural features and phylogenetic relationships. Carbohydr Res 339:2281–2295
Marquez J, Seoane-Camba JA, Suárez-Cervera M (1997) Allergenic and antigenic proteins released in the apertural sporoderm during the activation process in grass pollen grains. Sex Plant Reprod 10:269–278
Mascarenhas JP (1993) Molecular mechanisms of pollen tube growth and differentiation. Plant Cell 5:1303–1314
Miller WB, Ranwala AP (1994) Characterization of three soluble invertase forms from Lilium longiflorum flower buds. Physiol Plant 92:247–253
Mollet JC, Park SY, Nothnagel EA, Lord EM (2000) A lily stylar pectin is necessary for pollen tube adhesion to an in vitro stylar matrix. Plant Cell 12:1737–1749
Nebenführ A, Staehelin LA (2001) Mobile factories: Golgi dynamics in plant cells. Trends Plant Sci 6:160–167
Ohtsuki T, Taniguchi Y, Kohno K, Fukuda S, Usui M, Kurimoto M (1995) Cry j 2, a major allergen of Japanese cedar pollen, shows polymethylgalacturonase activity. Allergy 50:90–93
Parre E, Geitmann A (2005) Pectin and the role of the physical properties of the cell wall in pollen tube growth of Solanum chacoense. Planta 220:582–592
Pressey R, Reger BJ (1989) Polygalacturonase in pollen from corn and other grasses. Plant Sci 59:57–62
Raposo G, Kleijmeer MJ, Posthuma G, Slot JW, Geuze HJ (1997) Immunolabelling of ultrathin cryosections: application in immunology. In: Herzenberg LA, Weir D, Blackwell C (eds) Handbook of Experimental Immunology vol. 4. Blackwell Science Inc, Cambridge, pp. 1–11
Roberts-Oehlschlanger SL, Dunwell JM, Faulks R (1990) Changes in the sugar content of barley anthers during culture on different carbohydrates. Plant Cell Tiss Org Cult 22:77–85
Saini HS (1997). Effects of water stress on male gametophyte development in plants. Sex Plant Reprod 10:67–73
Saini HS, Lalonde S (1998) Injuries to reproductive development under water stress, and their consequences for crop productivity. J Crop Prod 1:223–248
Singh MB, Knox RB (1984) Invertases of Lilium pollen. Plant Physiol 74:510–515
Speranza A, Calzoni GL, Pacini E (1997) Occurrence of mono- or disaccharides and polysaccharide reserves in mature pollen grains. Sex Plant Reprod 10:110–115
Stadler R, Truernit E, Gahrtz M, Sauer N (1999) The AtSUC1 sucrose carrier may represent the osmotic driving force for anther dehiscence and pollen tube growth in Arabidopsis. Plant J 19:269–278
Staehelin LA, Moore I (1995) The plant Golgi apparatus: Structure, functional organization and trafficking mechanisms. Ann Rev Plant Physiol Plant Mol Biol 46:162–288
Suárez-Cervera M, Marquez J, Seoane-Camba JA (1995) Pollen grain and Ubisch body development in Platanus acerifolia. Rev Palaeobot Palynol 85:63–84
Suárez-Cervera M, Arcalís E, Le Thomas A, Seoane-Camba JA (2002) Pectin distribution pattern in the apertural intine of Euphorbia peplus L. (Euphorbiaceae) pollen. Sex Plant Reprod 14:291–298
Suárez-Cervera M, Takahashi Y, Vega-Maray AM, Seoane-Camba JA (2003) Immunocytochemical localization of Cry j 1, the major allergen of Cryptomeria japonica (Taxodiaceae) in Cupressus arizonica and Cupressus sempervirens (Cupressaceae) pollen grains. Sex Plant Reprod 16:9–15
SWISS-PROT: Q6H9K0 Exopolygalacturonase precursor (Pollen allergen Pla a 2). http://www.ncbi.nlm.nih.gov/entrez/viewer.fcgi?db=protein&val=51316214
SWISS-PROT: Q8GT41 Putative invertase inhibitor precursor (Pollen allergen Pla a 1). http://www.ncbi.nlm.nih.gov/entrez/viewer.fcgi?db=protein&val=29839547
Swoboda I, Grote M, Verdino P, Keller W, Sing MB, De Weerd N, Sperr WR, Valent P, Balic N, Reichelt R, Suck R, Fiebing H, Valenta R, Spitzauer S (2004) Molecular characterization of polygalacturonases as grass pollen-specific marker allergens: expulsion from pollen via submicronic respirable particles. J Immunol 172:6490–6500
Taniguchi Y, Ono A, Sawatani M, Nanba M, Kohno K, Usui M, Kurimoto M, Matuhasi T (1995) Cry j 1, a major allergen of Japanese cedar pollen, has pectate lyase enzyme activity. Allergy 50:90–93
Vega-Maray A, Fernández-González D, Valencia-Barrera R, Seoane-Camba JA, Suárez-Cervera M (2004) Lipid transfer proteins in Parietaria judaica L. pollen grains: immunocytochemical localization and function. Eur J Cell Biol 83:493–497
Wobus U, Weber H (1999) Sugars as signal molecules in plant seed development. Biol Chem 380:937–944
Wolters-Arts M, Lush WM, Mariani C (1998) Lipids are required for directional pollen tube growth. Nature 392:819–921
Ylstra B, Garrido D, Busscher J, van Tunen AJ (1998) Hexose transport in growing Petunia pollen tubes and characterization a pollen-specific, putative monosaccharide transporter. Plant Physiol 118:297–304
Zhang GF, Staehelin LA (1992) Functional compartmentation of Golgi apparatus of plant cell. Immunocytochemical analyses of high pressure frozen and freeze substituted sycamore maple suspension culture cells. Plant Physiol 99:1070–1083
Acknowledgements
The authors are grateful to the Scientific Technical Services of the University of Barcelona for their careful preparation of samples for TEM and SEM. This study was supported by grants BOS2000–0563 and BOS2003–06329, Department of Science and Technology, Spain, and also by grant Russian Foundation of Fundamental Research, RFFI no. 03–04–49108 for Nina Gabarayeva.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Suárez-Cervera, M., Asturias, J.A., Vega-Maray, A. et al. The role of allergenic proteins Pla a 1 and Pla a 2 in the germination of Platanus acerifolia pollen grains. Sex Plant Reprod 18, 101–112 (2005). https://doi.org/10.1007/s00497-005-0002-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00497-005-0002-4