Abstract
Key message
The rate of vessel tapering is highly conserved across a precipitation gradient in tropical trees, pointing to a limit on tree height determined by a maximum basal vessel diameter.
Maximum tree height in the tropics decreases strongly with decreasing precipitation. The role of hydraulic architecture in controlling this variation in tree height remains unclear. The widening of conducting xylem vessels from the apex to the base of trees, also known as tapering, is important for maintaining the hydraulic conductivity along the tree stem. If in contrast vessel diameter were constant, then resistance would increase with path length constraining flow rates as tree height increases. Whilst previous research has shown that vessel diameter scales with tree height at a similar rate (similar power law exponent) across biomes and taxa, knowledge on these relationships across precipitation gradients within a single species is incomplete, especially for the tropics. Here we report how vessel density and diameter at the tree base differ for two tropical Cedrela species across four sites varying in precipitation from 1014 to 2585 mm year−1. We find that maximum tree height decreases with decreasing precipitation across sites from 42 to 13 m. Despite the strong differences between sites in maximum tree height and water availability, tapering is indeed remarkably conserved and close to published scaling with height based on multi-species analyses. Thus, for a given tree height, basal vessel diameter does not vary between sites, whilst the maximum basal vessel size is two times smaller at the drier site (with the shortest trees) compared to the wettest site (with the tallest trees). This suggests a possible limitation of tree height determined by a maximum basal vessel diameter that can be sustained, given increasing embolism risk with increasing dryness. Our results show no hydraulic adaptation across this wetness gradient and reveal a clear relationship between maximum tree height and maximum basal vessel size.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Trees need light to grow and function, and thus strive to reach the canopy to optimise light capture in forests. At the same time, trees need to keep their leaves well-watered, requiring transport of water to the canopy against gravity and friction. It has therefore been hypothesised that tree height might be limited by frictional losses and gravity (Ryan and Yoder 1997). Evidence for this hypothesis comes in part from patterns of increasing maximum tree height with water availability, suggesting a strong role for water availability in the limitation of maximum height (Tao et al. 2016; Liu et al. 2019).
Water flow from the roots to treetop is driven by pull (negative pressure) from the leaf exerted on the water column. The xylem water transport network can be considered as a series of interconnected tubes that branch from the base of the tree trunk to provide water to the leaves (Tyree and Ewers 1991). One of the main impediments to water flow is resistance, Z, caused by friction which decreases with the fourth power of the vessel diameter, according to Hagen–Poiseuille's law, i.e.
According to this equation (modified for a single conduit from Tyree and Ewers (1991)), the increase in resistance with greater path length, l (i.e. increase in tree height), for a fluid with viscosity η can be counteracted by increasing vessel diameter, d. Hence, one strategy for trees to reduce the frictional constraint on tree height is to steadily widen vessel diameter from the top of the tree towards its base. To permit constant volume flow and supply a branching canopy, vessels will have to divide accordingly from base to tip. Based on these principles, it has been shown that total frictional resistance can be regulated to be independent of path length through coordinated changes in vessel division and vessel diameter with tree height (Savage et al. 2010; West et al. 1999). The pattern of widening and combining of xylem vessels from tree apex to the base is also known as tapering.
Water under highly negative pressures in the xylem is in a metastable state that makes spontaneous cavitation and heterogeneous air bubble nucleation possible (Knipfer et al. 2015; Tyree and Sperry 1989). More negative water potentials are associated with greater risk of gas bubble formation and spread (Sperry 1986; Sperry and Love 2015; Sperry and Tyree 1988; Tyree and Sperry 1989). If these air bubbles, known as embolisms, become large enough, they may reduce or block water flow to the leaves, or even result in a complete loss of conductivity, culminating in dehydration and tree death (Adams et al. 2017; Brodribb and Cochard 2009; Hammond et al. 2019). This is likely to be a major mechanism of tree death during droughts (Adams et al. 2017), since lower soil water potential requires more negative pressures to induce water flow (Vilagrosa et al. 2012). Taller trees that make wider vessels to counterbalance resistance are likely to be more vulnerable to embolism (Olson et al. 2018; Scoffoni et al. 2017; Sperry et al. 2006), and trees thus face a trade-off between producing wider vessels to increase conductance and greater vulnerability to embolism (Levionnois et al. 2021; Lobo et al. 2018; Olson et al. 2018; Scoffoni et al. 2017; Sperry et al. 2006). Whilst there is some debate about whether embolism occurs in the trunks of tall trees, Wason et al. (2018) show that water potentials may become low enough in the trunks of canopy trees to cause air seeding in some species. Furthermore, Guan et al. 2022 showed little difference between leaves and stems in their hydraulic safety margins, thereby suggesting that stems are as likely to form embolism as more apical tissues. Observations suggest that tall trees suffer indeed higher mortality during droughts (Bennett et al. 2015; Johnson et al. 2018b; Stovall et al. 2019), possibly due to greater occurrences of embolism. Thus, climate change-associated droughts may decrease forest height in the future (Anderegg et al. 2019; Fajardo et al. 2019; Shenkin et al. 2018; Stovall et al. 2019).
Tapering is well documented in trees, with evidence that trees tend to optimise water conductance by offsetting frictional losses through vessel widening (Anfodillo et al. 2006; Olson et al. 2014; Petit et al. 2010). Building on the work of West et al. (1999), Enquist (2002, 2003) suggested that biomechanical constraints and the need to reduce path flow resistance should lead to universal tapering, or in other words, the rate of vessel diameter decrease per unit height increase from the tree base to apex should be the same across trees and taxa. Hydraulic optimality models predict that xylem vessel diameter, D, tapers up trees with distance, H, from the apex following a power law D = Hα with a scaling exponent α = 0.2 (i.e. a linear relationship between log(D) and log(H) with a slope of 0.2) (Anfodillo et al. 2006; Enquist 2003; Savage et al. 2010; West et al. 1999). Vessel widening at a rate lower than the scaling exponent of 0.2 results in large hydraulic resistance increases with path length, whilst tapering above this optimal value results in relatively small gains in resistance reduction and disproportionate increases in risk to embolism and reduced mechanical strength due to larger basal vessel diameters (Christensen-Dalsgaard et al. 2007; Fan et al. 2017; Knipfer et al. 2015; Olson et al. 2018; Savage et al. 2010; Scoffoni et al. 2017; Sperry et al. 2006). Multi-taxa studies show that a fundamental, universal scaling relationship exists between vessel diameter and tree height across ecosystems and climates (Olson et al. 2014). This suggests that this trait is fixed or invariant with respect to climate, but few studies have investigated how vessel diameter changes with tree height across large water availability gradients within single species.
It is still little known to what degree the relationship between vessel diameter and tree height is an evolutionary adaptation, and whether this relationship is conserved within a species growing under very different water availabilities. This has implications for how trees cope with changes in water availability and hydraulic stress associated with climate change, and affect the ability of trees to grow taller and how mortality risks increase with tree height (Anderegg et al. 2012; Rosell et al. 2017). This study provides one of the first assessments of how trees adjust hydraulic architecture in response to variation in water availability within a widespread tropical tree species. Some recent studies have looked at the effect of water availability on hydraulic architecture in tropical trees, but these include only relatively short trees (Liang et al. 2019), or only young (5 year) even-aged plantation trees (Ramesha et al. 2022), and do not explicitly analyse relationships with tree height from small saplings to maximum height per site. We propose three different strategies (A – C below and Fig. 1) as to how trees may adjust xylem vessel diameter across differences in water availability:
-
(A)
Equal tapering across sites, but different basal vessel size for a given tree height. For a given tree height, trees at drier sites have vessels that are less conductive compared to those at wetter sites. Trees prioritise safety against water transport efficiency under drier conditions and thus produce smaller basal vessels at the drier sites at the cost of lowering maximum flow rates.
-
(B)
Equal taper rate and basal vessel size for a given tree height irrespective of water availability. Hydraulic architecture does not change in response to changes in water availability, and at a given height trees growing at drier sites will thus experience greater hydraulic stress and embolism risk when compared to trees at wetter sites.
-
(C)
Reduced taper rate to reduce basal vessel size. For a given tree height, trees at drier sites have identical apical vessel diameter, but smaller basal vessels and thus reduced embolism risk but higher resistance and thus lower conductivity.
Conceptual illustration of three possible hydraulic strategies of trees in response to water availability. Upper panels show the expected log–log relationships between vessel diameter and tree height at wet (blue) and dry (red) sites. Lower panels show a schematic diagram of the vessel tapering along the tree stem and hypothetical difference in basal and apical vessel diameters between dry and wet sites. See main text for description of the two strategies. Note that the expected log–log relationship in the lower panels shows as linear vessel tapering. This present study analyses the relationship between basal vessel diameter and tree height. The log–log relationships in the upper panel between vessel diameter and distance from apex can represent similarly the relationship between basal vessel diameterand total tree height (as measured here), or the change in vessel diameters vertically from tip to base ( as done by Fajardo et al. 2020; Olson et al. 2014)
The first strategy, equal tapering across sites but different basal vessel size for a given tree height, has previously been suggested as a mechanism of trees to cope with increasingly hydraulically stressful growing conditions (Enquist 2002; Rosell et al. 2017). As competition for light decreases, trees could prioritise traits designed to deal with water stress (i.e. make narrower and safer vessels) above traits that allow faster growth (i.e. greater hydraulic conductivity) (Brenes-Arguedas et al. 2011; Markesteijn et al. 2011). Evidence for this strategy has been observed in the widespread Australian genus Eucalyptus, where species growing in drier environments tend to have reduced basal vessel diameter for a given tree height relative to congeneric species in wetter sites, likely driven by genetic variation between species (Pfautsch et al. 2016). Support for the second strategy, equal taper rate and vessel size (B), comes from recent studies assessing the role of climate on vessel anatomy within species. These show that tapering amongst wet and dry sites is similar and thus for a given height average vessel diameter is the same (Avakoudjo et al 2022; Fajardo et al. 2020; Garcia-Cervigon et al. 2018; Lechthaler et al. 2019; Pfautsch et al. 2016; Warwick et al. 2017). One recent study showed vessel diameter information in two species across a precipitation gradient in temperate southern Chile at a fixed distance from the apex in trees of fixed height (Garcia-Cervigon et al. 2018). They showed that in Embothrium coccineum vessel diameter at fixed distance from apex increased from dry to wet conditions (suggesting A or C depending upon whether vessel diameter at the apex is fixed or not), whereas in Nothofagus antarctica no significant increase was observed (suggesting scenario B). Similar to the results presented for Embothrium coccineum, Liang et al. (2019) showed that for Castanopsis fargesii across a broad precipitation gradient vessel diameter at a fixed point below the apex and at fixed tree height increased from wet to dry conditions (thus suggesting either scenario A or C).
Strategy C allows for different vessel scaling dependent on tree height for the same species in response to differences in water availability. To our knowledge, there is little evidence for differing tapering rate across climates, either within or between species. Similar vessel scaling with tree height appears to be a universal property of trees, but species may deviate from the general rule. Recent research has shown that across short-rotation plantations of Melia dubia, trees grown in wet sites versus dry sites had similar apical vessel diameter but larger stem vessel diameter, after controlling for path length (Ramesha et al. 2022). This hints at different rates of vessel widening from the apex across water availability gradients (i.e. scenario C), though notably the authors obscure their findings with regards to vessel scaling.
We here test which tapering strategy is observed in two congeneric species in the tropical genus Cedrela. We use wood anatomical analyses to study how xylem vessel size and density are related to tree height and change along a water availability gradient across four sites in South and Central America. Vessel anatomy–tree height relationships across broad climate gradients within taxa can provide insights to what degree trees can adapt vessel anatomy to different growing conditions, and could help explain variation in maximum tree height along gradients of water availability (Fajardo et al. 2019; Moles et al. 2009; Rosell et al. 2017; Scheffer et al. 2018; Tao et al. 2016).
Methods
Species
Cedrela is a widespread neo-tropical tree genus in the Meliaceae family. In this study, two species were sampled: C. odorata (L.), found throughout the neotropics, and C. salvadorensis (Standl.), restricted to Central America. C. odorata grows predominantly on well-drained soils at altitudes below 1300 m (Cintron 1990). Genetic studies of Cedrela have suggested that C. odorata may be polyphyletic, with a distinction between Central American C. odorata and South American C. odorata populations (Finch et al. 2019; Muellner et al. 2009, 2010). Cedrela trees are deciduous regardless of water availability and restrict growth to the wetter portion of the year, thus producing semi-ring-porous tree rings (Baker et al. 2017). C. odorata is a fast growing species (Brienen et al. 2010; Worbes 1999) with a relatively low wood density (0.32–0.35 g cm−3) occurring across a very broad precipitation range from a-seasonal wet tropical forest to highly seasonal dry forest (Baker et al. 2017; Gutierrez-Vazquez et al. 2012). It is able to attain heights of over 40 m, and can increase in height by more than 1 m per year in a wet tropical climate (Lamb 1968). It responds to low water availability by reducing radial growth (Worbes 1999). C. odorata is shallow rooted under most conditions and strongly relies on water from the upper 30 cm of soil (Cintron 1990; Schwendenmann et al. 2015).
Sites and sampling procedure
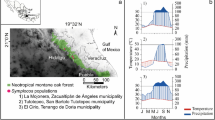
Cedrela trees were sampled at four sites (Fig. 2A), Oaxaca (Mexico, Cedrela salvadorensis), Yucatan (Mexico, Cedrela odorata), Selva Negra (Bolivia, Cedrela odorata), and Yasuni (Ecuador, Cedrela odorata). These sites span an aridity gradient characterised by a change in annual precipitation from 1014 to 2585 mm year−1, and change in length of dry season (months < 100 mm mo−1) from 8 to 0 months (Fig. 2C–F). Precipitation data are from one (for Selva Negra) or two weather stations closest to each site (Peterson and Vose 1997). For Oaxaca, the stations are Santiago Chivela (16.7 N, 95E) and Ixtepec (16.6 N, 95.1E), for Yucatan Xcupil (19.7 N, − 89.9E) and Champoton (19.4 N, − 90.7E), for Selva Negra, an unnamed station (9.72 N, 66.5E), and for Yasuni, Tiputini (− 0.8 N, − 75.4E) and Nuevo Rocafuerte (− 0.92 N, − 75.4E). Sites differ in soil type with well-drained karstic soils at the two driest sites (Table 1) and clay-based soils at the wetter two sites. Forests at the two driest sites in Mexico can be classified as seasonally dry forest (Perez-Garcia et al. 2010; White and Hood 2004), with the lowest water availability for trees in Oaxaca. At this site, mean annual precipitation is similar to that in Yucatan, but precipitation is concentrated over fewer months (Fig. 2) and occurs in few high intensity events associated with hurricane activity (Brienen et al. 2013). In addition, trees at this site grow on steep karstic slopes, resulting in a xerophytic, low stature vegetation (Perez-Garcia et al. 2010).
Location of sampling sites (A), diameter-height allometries at the sampling sites (B), and site monthly precipitation (C–F) with mean annual precipitation in red text (mm) and mean monthly temperature as the red line. Climate data were taken from an average of the two closest climate recording stations to each site, except for Selva Negra, which had only one close climate recording station (Peterson and Vose 1997)
For each tree, we measured tree diameter and estimated tree height by eye. Whilst this method is prone to errors and biases, we attempted to limit those errors using two independent estimates from experienced forest scientists. Note that the measurement error is likely larger for tall trees (~ 5 m) than for short trees (~ 1 m). Additionally, we compared tree height estimates by eye with tree height measured in felled trees for a similar tropical forest site in Bolivia, where DBH–height relationships were shown to be very similar for both methods (see supplementary Fig. 3). The maximum tree height decreased from 42 m in Yasuni, the wettest site, to 13 m at the driest site in Oaxaca (Table 1). All sites are old growth forests although two of the sites (Selva Negra and Yucatan) have experienced selective logging in the past of less than one tree per hectare.
To measure how xylem vessel diameter and density varies across sites and tree heights we used tree cores taken from the base of trees in vivo, or stem disc sections from the bases of felled trees or from small saplings (Brienen et al. 2010). We used a 5.15 mm borer at the Oaxaca site and 10 mm increment borers at the wetter three sites. The use of different borer diameters was due availability at the time of sampling. At each site, trees covering the full range of heights were sampled (see Table 1). Samples were generally taken close to breast height (1.3 m). From here on, ‘tree height’ refers to the height of the tree minus the height above ground at which the sample was taken.
For estimating how basal vessel diameter and density changes with tree height, we adopted two approaches (see Fig. 3). In the first method, the inter-tree sampling approach, we measured vessel diameter and density only in the outer 2 cm of wood at the base of different trees of known heights. In the second, the intra-tree approach, we sampled 2 cm long radial increments at least 2 cm apart from each other along a radius of a stem cross section (disc) or core. The number of samples analysed for each core or disc depended upon tree diameter: trees with large diameters were sampled at less regular distance with spacing of up to 8 cm between sampled radial sections compared to smaller trees. This is justified as DBH–height allometry is not linear and large trees change less in tree height compared to smaller trees (see DBH–height allometry plot in Fig. 3). For these samples from the intra-tree approach, the corresponding tree height at each sampling point was estimated using the site-specific diameter at breast height (DBH)—– tree height allometry (see Fig. 3 for estimates of DBH allometry which gives estimated height for a radial position). This intra-tree approach assumes that vessel diameter for a given height does not vary over time due to, for example, climate change or increases in [CO2]. Fajardo et al. (2020) showed that scaling relationships are similar across individuals and within individuals. In line with this, we found that vessel diameter measurements did not differ between the intra- and inter-tree approaches (see Fig. S1), and thus in our main analysis, we merged the two datasets. Sample sizes for both methods are shown in Table 1.
Schematic of two sampling approaches to assess the effect of tree height on vessel anatomy. In the inter-tree approach, we measured vessel diameter and density only in the outer wood at the base of different trees of known heights (hatched boxes). In the intra-tree approach, we sampled sections along the stem cross section at the base of trees at increasing distances from the tree pith within individual trees (open boxes). This results in within-tree ontogenetic patterns of changes in vessel anatomy with height, where we derived height at various sections using site allometric relationships between tree diameter and height (see Fig. 2B, allometry for all sites). Left hand photographs show the surface of Cedrela tree cores that were used for image analysis, the scale bar is 1 mm
Wood cores were glued on wooden frames and either sanded or cut with a microtome blade to aid vessel visualisation. Photographs were taken of each wood section under a microscope using a Canon EOS1100D camera with 2 × magnification power lens using a Leica S6E microscope at 2 × magnification power (4 × for small trees). The digital images used for analysis had resolutions of 72 dpi, equivalent to 1.17 µm per pixel at 2 × magnification. A stage micrometre was included in the images to provide a length scale. Xylem vessels were identified manually on photographs and their area was measured using digital software ImageJ (Fiji, version 1.52p). On average, this was done for over 100 vessels per image (see Fig. S2). A minimum of 50 vessels were recommended for sufficient statistical power considering vessel diameter variability in ring-porous species (Scholz et al. 2013). Vessel diameter was calculated from vessel area assuming vessels were circular, which was largely the case. For each wood section, vessel density was calculated as the mean number of vessels per unit area using the full area of the image.
Analysis
In agreement with existing studies, we found that the relationships between the dependent variables, vessel diameter (D) and density (d) with the independent variable, tree height (H), follow a power law (i.e.\(D\propto {H}^{\alpha }\)), where α is the power law exponent. To examine further how the basal vessel diameter and density tree height relationships varied between sites, we used linear mixed effects models (LMMs) of the following form:
Here μ0i is the random intercept for each individual tree i and εi is the residual error for each individual tree. We incorporated a random intercept for individual trees since 74 individual trees were sampled more than once (i.e. along the stem disc or tree core, intra-tree approach, see Fig. 3). Inclusion of these random intercepts improved the model fit according to AIC values, whilst inclusion of random slopes (for each tree) did not improve the fit. Note that slopes were similar for the two sampling approaches (i.e. inter and intra-tree sampling, see supplementary Fig. 1) justifying the use of one single statistical model for the two datasets. We log-transformed vessel diameter and vessel density to satisfy the assumption of linearity, as well as to permit comparison with other studies (Olson et al. 2014). We used the lme4 R package to produce the LMMs (Bates et al. 2015). Pseudo R2 values were estimated for LMMs to assess the variance explained by the model, with and without the random effects as per Nakagawa and Schielzeth (2013) using the MuMIn R package (Barton 2019). If the interaction effect of site with height is not significant, then slopes between sites can be considered similar. Bonferroni corrected t tests were then carried out to determine if the intercepts differed between sites, using the R package emmeans (Lenth 2018). All linear models were assessed for homogeneity of slopes using the ANOVA R function, normality of the residuals (Shapiro–Wilk from the R package rstatix (Kassambara 2019) and Q–Q plots) and homogeneity of variance (Bartlett test from the R package stats; R Core Team 2018).
We also tested if there was a difference between sites in mean vessel diameter and density of the 5 tallest trees. In addition, we tested if there were differences between sites in mean vessel diameter and density for a given height by selecting only trees between 2.5 and 7.5 m tall. Mean height varied slightly between sites, between 3.14 m and 5.43 m, see Table S3). Non-parametric tests (Kruskal–Wallis, and Wilcoxon signed-rank tests) were used since these subsets of the data were not normally distributed. P values were adjusted for multiple comparisons using Bonferroni correction. All analyses were performed in RStudio using R version 3.5.1 (R Core Team 2018).
Results
The increase in mean basal vessel diameter with tree height did not differ significantly between the four different sites (i.e. slopes in mixed effects model were similar and there was no significant interaction between height and site, see Fig. 4A, B and Table 2). The model explained a high proportion of the variance in vessel diameter across samples. Intercepts of the model indicated that mean basal vessel diameter for a given height only differed significantly (p = 0.006) between Selva Negra and Yucatan with 1.763 times greater basal vessel diameters in Yucatan than in Selva Negra (Table 2). Despite this difference, there was no evidence that trees in drier sites have consistently larger or smaller vessels for a given height than trees in wet sites.
Relationships between mean basal vessel diameter (A, B) and basal vessel density, and tree height (C, D). A and C show log-transformed data with linear regression lines for each site (for coefficients and comparisons of intercepts see Table 2). Panels B and D show non-transformed data, with regression equations of the form resulting in y = const xα taken from the logged linear relationship coefficients of (A, C)
In contrast to basal vessel diameter, we find that basal vessel density decreased with tree height at a different rate for the different sites (Table 2). For trees at the driest site, Oaxaca, vessel density declined more rapidly with tree height (− 0.876 log mm per log m) compared to the other three sites (− 0.53 to − 0.64 log mm per m) (Fig. 4C, D, Table 2). The wetter three sites, all with C. odorata, share similar declines in vessel density with height (Table 2) and have similar intercepts with no indication for drier sites to have lower or higher vessel density (Table 2). The LMM accounted for a high proportion of the variance in vessel density across samples, with the fixed effects alone accounting for 84% of total variation and the model including random effects accounting for 91%.
Finally, we tested for differences between sites in basal vessel diameter and density of small, similarly sized trees (ca. 5 m tall) and for the tallest trees. This shows that whilst vessel density did not differ between trees of similar size, the mean vessel diameter of the tallest trees increased with site wetness, nearly doubling between Oaxaca and Yasuni (Fig. 5A, C). For basal vessel density, we also find no differences in trees of similar size, but in the tallest trees, we find significantly lower density in wet sites relative to drier sites (P < 0.05) (Fig. 5B, D).
Mean vessel diameter (A, C) and vessel density (B, D) for 5 m tall trees (A, B) and the 5 tallest trees per site (C, D). Points represent the mean vessel diameter per sample in (A, C). Points represent the number of vessels per measured area of each sample in (B, D). Differences between sites is shown in the plots using a Kruskal–Wallis test, as is significance of pairwise comparisons using Wilcoxon rank-sum tests. Adjusted p values are shown: ns: not significant (p > 0.05), *:p < 0.05,**:p < 0.005
Discussion
A basic question is how, within one tropical tree species, tree hydraulic architecture varies across a strong water availability gradient. This question may help understanding mechanisms responsible for observed variation in maximum tree height with water availability (Fajardo et al. 2019; Moles et al. 2009; Rosell et al. 2017; Scheffer et al. 2018; Tao et al. 2016), but has to our knowledge not yet widely been examined in tropical trees. Our focus on these relationships for a single species (or genus) reduces the number of unknowns that are inherent in multi-species synthesis studies. We assessed how tree hydraulic architecture varies across a water availability gradient within the widespread and important tropical genus Cedrela. We find that taper rate and basal vessel size of Cedrela do not vary across sites, corresponding to the strategy B in Fig. 1. Thus, variation in basal vessel size is entirely controlled by tree height, and does not vary with climate. This invariance in the basal vessel size–tree height relationship across the water availability gradient in our study is consistent with findings from two temperate tree species across strong precipitation gradients (Fajardo et al. 2020), and more generally with the observation that tree size is a better predictor for basal vessel size than climate across a range of tree species and tree sizes (Olson et al. 2014).
Interestingly, whilst this constancy of taper rates across sites does not result in differences in basal vessel diameter for a given tree height (Fig. 5A), we find that the maximum basal vessel size of the tallest trees is two times smaller at the drier site compared to the wettest site (Fig. 5C). These differences are explained by a general decrease in maximum tree height from the wettest to the driest sites, from 42.5 to 13 m (Fig. 2B) and not by a change in the relationships between tree height and vessel size (Fig. 4) for different sites. A possible explanation for this lack of taller trees at drier sites is that lower water availability pushes taller trees at these sites beyond the hydraulic limits of what can be sustained with a (given) basal vessel diameter. Hydraulic failure risk via embolism is generally thought to increase as vessel diameter increases leading to increases in embolism risk as tree height increases (Levionnois et al. 2021; Lobo et al. 2018; Olson et al. 2018; Prendin et al. 2018; Scoffoni et al. 2017; Sperry et al. 2006). This points towards a role of basal vessel diameter, and related embolism risk, in controlling maximum tree height. Vessel widening as trees get taller causes trees at dry sites to reach their critical threshold for embolism at shorter tree height, as xylem water potentials are lower for dry sites due to lower soil water availability. In contrast, higher xylem water potentials at wetter sites allow much greater vessel diameter—and thus tree height—for the same embolism risk. This mechanism could explain the decrease in tree height across sites as well as the strong decrease in tree longevity from 308 years at the wettest to 117 years at the driest site (Unpublished data, R. Brienen; Unpublished data, P. Groenendijk; Brienen et al. 2010). This possible mechanism of hydraulics controlling tree height and longevity across water availability gradients suggested here for Cedrela may also be responsible for widespread observations of reductions in tree height and longevity with increasing aridity in the tropics (for tree height see Klein et al. 2015; Tao et al. 2016, and for tree longevity, see Locosselli et al. 2020).
Despite a debate regarding the extent to which vessel diameter affects embolism vulnerability (Gleason et al. 2016; Liu et al. 2020), various lines of evidence point towards links between vessel diameter and embolism and eventually mortality. Measurements of embolism risk of planted saplings with height up to 4 m for three species reveal a decrease of the water potential at 50% stem conductivity loss with tree height (Olson et al. 2014). This latter finding is consistent with the interpretation of our results, that basal vessel diameter depends on tree height but not climate, and thus that increased mortality risk at drier sites is probably related to tree hydraulics. This interpretation is also consistent with the results of a study by Shenkin et al. (2018) who investigated the effect of the 2004/5 El Nino drought event on tree mortality in natural forests in Bolivia. These authors found strongest increases of mortality risk with increasing drought stress in tall trees and attribute the result to tree hydraulics. Increased risk of tall trees under drought stress has also been found in global meta-analyses (Bennett et al. 2015; Johnson et al. 2018b), and a long-term Amazon drought experiment (Rowland et al. 2015).
What these studies cannot discern, however, is to what extent increased mortality is indeed related to embolism risk in the stem or rather the roots, or due to different drought-related deleterious effects (Brodribb and Cochard 2009; Brunner et al. 2015; McDowell et al. 2008). We can of course also not rule out hydraulic adaptations that may offset the decreases in water availability across sites and mitigate drought stress. For example, trees may vary their inter-vessel pit architecture which has been shown to vary across species (Lens et al. 2016), and to a limited degree within species (Kotowska et al. 2020), and could result in decreases in embolism risk (Medeiros et al. 2019; Pittermann et al. 2010). In addition, trees may increase investment in root tissue and rooting depth (Brum et al. 2019; Dawson and Pate 1996), or trees may reduce the effect of hydraulic stress through strict control of stomatal conductance (i.e. isohydry) (Jones 1998; Tardieu and Simonneau 1998), or by shedding leaves to avoid excessive water loss (Manzoni et al. 2015; Vico et al. 2017). Cedrela trees are indeed likely to be at least to some extent isohydric given their relatively large vessels and high water potential at which 50% loss of conductance occurs (> − 1 MPa stem p50), and high osmotic potential at which leaves lose their turgor (> − 1.4 MPa) (Hoeber et al. 2014; Villagra et al. 2013a, b). Cedrela is also a deciduous tree species, thereby limiting exposure to dangerously low water potentials during the dry season. Nonetheless, trees at the drier site are likely to experience greater hydraulic stress more frequently than trees of equivalent height at the wet site, due to lower wet season rainfall levels (see Fig. 2 and Table 2) and fast draining karstic soils leading to very low soil water potentials. This is confirmed by analysis of tree ring and carbon isotope data from the same sites which shows that diameter growth of Cedrela odorata trees in the dry Yucatan site is more strongly limited by water availability than trees growing at the wetter site in Bolivia (Brienen et al. 2022).
We also show that for Cedrela trees vessel density decreases as vessel diameter increases with tree height. This is expected from tapering theory as vessels divide and become smaller, increasing in vessel density (Savage et al. 2010; West et al. 1999). Vessel density increases may be able to offset losses in conductance due to decreases in vessel diameter, thus providing a potential strategy to avoid embolism risk, whilst maintaining high conductance (Echeverria et al. 2019). Therefore, we may have expected that trees in drier sites would have higher vessel density and smaller vessels. This does not appear to be the case in Cedrela across the broad climate gradient covered in this study. C. salvadorensis trees from Oaxaca decrease in vessel density at a higher rate than the wetter three sites. This may, however, be due to inter-species variation, rather than any climatic influence on vessel density per se.
The universal scaling of vessel diameter
Using global multi-species data, Olson et al. (2014) found a mean slope of 0.46 (95% confidence interval of 0.41–0.51) for the relationship between log basal vessel diameter and log tree height. Our slope values are very similar to slightly lower (0.39–0.47, Table 2) compared to the global data. The slopes of the relationship between log basal vessel density and log tree height found here (− 0.53 to − 0.88) are also largely within the range of the values (0.73, 95% confidence interval of 0.86–0.61) reported by Olson et al. (2014). Because of the global nature of their study they suggested that regardless of growing conditions trees should achieve similar rates of increase in vessel diameter with tree height. Our study and the recent study of Fajardo et al. (2020) show this also to be the case within species across a gradient of very different water availabilities. Together, these results suggest that the underlying mechanism behind the process of vessel production at different heights is likely highly conserved in evolution.
It is unclear how trees of very different taxonomy and growing in very different climate and soils show such similarity in their increase in basal vessel diameter with height. Several possible mechanisms have been presented to explain this constant scaling of vessel diameter with height (Fajardo et al. 2020). One hypothesis proposed by Woodruff and Meinzer (2011) and later discussed by Fajardo et al. (2020) is that vessel diameter is controlled by the turgor pressure at the site and time of the formation of the vessel. Their hypothesis suggests the turgor pressure is in turn controlled by height, with the xylem water potential being more negative at greater heights, which also likely limits embolism risk further down the tree where vessels are wider. Our and Fajardo et al.'s (2020) data show that basal vessel diameters for trees of a given height are similar across climates whilst different xylem pressures are likely to differ given difference in soil water availability. It is therefore unlikely that this could explain constant scaling of vessel diameter with tree height.
Additionally, the observed increase of vessel size towards the base may be the result of a hormonal control. Anfodillo et al. (2012) found that increases in xylem conduit width with distance from apex in a conifer is caused by longer duration of cell expansion, which was hypothesised to result from a gradient in the growth hormone auxin from apex to base. In support of this, Hacke et al. (2017) found that variation in vessel diameter for a given height is likely mediated by endogenous, hormonal stimulation of cell growth, and Johnson et al. (2018a) found that treatment of Populus trees with an auxin transport inhibitor caused the formation of shorter vessels of comparably narrower diameter. However, auxin transport is also affected by drought (Korver et al. 2018), which would lead to differences in tapering across a climate gradient, and is thus not consistent with our results.
Whilst the ultimate mechanistic process behind tapering remains unresolved it is likely that the universal scaling of vessel diameter with tree height is the result of natural selection due to a cost–benefit trade-off. This trade-off is likely to be between small vessels with low flow rates for a given body size impeding photosynthesis and thus productivity, and large vessels that have innate vulnerability to embolism (Knipfer et al. 2015; Olson et al. 2018; Scoffoni et al. 2017; Sperry et al. 2006) and which may reduce mechanical strength (Christensen-Dalsgaard et al. 2007; Fan et al. 2017).
The rate of vessel tapering is highly conserved across taxa, but vessel diameter for a given height varies between related species (Lechthaler et al. 2019; Lens et al. 2004; Pfautsch et al. 2016). Plasticity in the diameter of vessels produced has been demonstrated in the short term in response to climate, similar to the effect of drought on tree ring width (Zweifel et al. 2006). Using tree-ring data in two tropical species, Locosselli et al. (2013) showed that vessel area is positively correlated with precipitation and negatively with temperature. Similar results were also obtained using tree-ring chronologies of Tectona grandis (Pumijumnong and Park 1999), and across drought periods in Chukrasia tabularis in Bangladesh (Islam et al. 2019).
Thus, it is conceivable that trees can alter their rate of tapering via endogenous stimulation in the short term, but the costs of doing so over long periods of time outweigh the benefits. Regardless of some evidence for short term plasticity in vessel characteristics, our results and existing literature show that the diameter and density of vessels for a given height appear highly conserved within species (Fajardo et al. 2020; Lechthaler et al. 2019; Pfautsch et al. 2016; Warwick et al. 2017), which suggests that most tree species cannot support long-term changes in vessel diameter and density for a given height due to risk of embolism or mechanical failure, and potential detrimental costs to the trees’ carbon balance. However, over evolutionary time scales, other adaptations in tree physiology that offset such costs may enable small differences in vessel diameter for a given height across related taxa higher than species (Lechthaler et al. 2019; Lens et al. 2004; Pfautsch et al. 2016).
The invariant nature of basal vessel widening in relation to rainfall, has consequences for the ability of trees to adapt to future changes in rainfall in the tropics. Our results indicate that water availability puts a limit on maximum basal vessel size, which in turn seems to play a role in controlling maximum tree height, as well as, tree longevity. Thus, rather than adjusting their principal hydraulic architecture, tropical trees growing in areas with decreasing rainfall are likely to see a reduction in their longevity and overall change towards shorter maximum tree height, consistent with observations of decreases in tree height (Klein et al. 2015; Tao et al. 2016) and tree longevity (Locosselli et al. 2020) with increasing dryness in the tropics.
Conclusion
We find that vessel diameter and density are remarkably similar for a given height, and that the rate at which basal vessel diameter scales with tree height (i.e. tapering) is remarkably conserved within species across sites varying in precipitation. Thus, trees do not adapt their hydraulic architecture to mediate decreases in water availability. We furthermore find that basal vessels of the tallest trees at the wettest site are almost two times greater than trees at the driest site, due to threefold difference in maximum tree height between sites. These results suggest that tree height is at least to some extent constrained by maximum basal vessel size, and indicate greater hydraulic vulnerability of similar sized trees at the drier sites. Our results could provide a mechanism to explain decreases in tree height and longevity with decreasing water availability as generally observed in the tropics.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Adams HD, Zeppel MJB, Anderegg WRL, Hartmann H, Landhausser SM, Tissue DT, Huxman TE, Hudson PJ, Franz TE, Allen CD, Anderegg LDL, Barron-Gafford GA, Beerling DJ, Breshears DD, Brodribb TJ, Bugmann H, Cobb RC, Collins AD, Dickman LT, Duan HL, Ewers BE, Galiano L, Galvez DA, Garcia-Forner N, Gaylord ML, Germino MJ, Gessler A, Hacke UG, Hakamada R, Hector A, Jenkins MW, Kane JM, Kolb TE, Law DJ, Lewis JD, Limousin JM, Love DM, Macalady AK, Martinez-Vilalta J, Mencuccini M, Mitchell PJ, Muss JD, O’Brien MJ, O’Grady AP, Pangle RE, Pinkard EA, Piper FI, Plaut JA, Pockman WT, Quirk J, Reinhardt K, Ripullone F, Ryan MG, Sala A, Sevanto S, Sperry JS, Vargas R, Vennetier M, Way DA, Xu CG, Yepez EA, McDowell NG (2017) A multi-species synthesis of physiological mechanisms in drought-induced tree mortality. Nat Ecol Evol 1:1285–1291
Anderegg WRL, Berry JA, Smith DD, Sperry JS, Anderegg LDL, Field CB (2012) The roles of hydraulic and carbon stress in a widespread climate-induced forest die-off. Proc Natl Acad Sci USA 109:233–237
Anderegg WRL, Anderegg LDL, Kerr KL, Trugman AT (2019) Widespread drought-induced tree mortality at dry range edges indicates that climate stress exceeds species’ compensating mechanisms. Global Change Biol 25:3793–3802
Anfodillo T, Carraro V, Carrer M, Fior C, Rossi S (2006) Convergent tapering of xylem conduits in different woody species. New Phytol 169:279–290
Anfodillo T, Deslauriers A, Menardi R, Tedoldi L, Petit G, Rossi S (2012) Widening of xylem conduits in a conifer tree depends on the longer time of cell expansion downwards along the stem. J Exp Bot 63:837–845
Avakoudjo HGG, Gebrekirstos A, Koné MW, Assogbadjo AE, Bräuning A (2022) Wood anatomy and vessel characteristics of spiny monkey orange (Strychnos spinosa) in Benin (West Africa). Dendrochronologia 72:125941. https://doi.org/10.1016/j.dendro.2022.125941
Baker JCA, Santos GM, Gloor M, Brienen RJW (2017) Does Cedrela always form annual rings? Testing ring periodicity across South America using radiocarbon dating. Trees-Struct Funct 31:1999–2009
Barton K (2019) MuMIn: Multi-Model Inference. R package version 1.43.15. https://CRAN.R-project.org/package=MuMIn
Bates DM, Martin BB, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Bennett AC, McDowell NG, Allen CD, Anderson-Teixeira KJ (2015) Larger trees suffer most during drought in forests worldwide. Nat Plants. https://doi.org/10.1038/nplants.2015.139
Brenes-Arguedas T, Roddy AB, Coley PD, Kursar TA (2011) Do differences in understory light contribute to species distributions along a tropical rainfall gradient? Oecologia 166:443–456
Brienen RJW, Zuidema PA, Martinez-Ramos M (2010) Attaining the canopy in dry and moist tropical forests: strong differences in tree growth trajectories reflect variation in growing conditions. Oecologia 163:485–496
Brienen RJW, Hietz P, Wanek W, Gloor M (2013) Oxygen isotopes in tree rings record variation in precipitation delta O-18 and amount effects in the south of Mexico. J Geophys Res Biogeosci 118:1604–1615
Brienen R, Helle G, Pons T, Boom A, Gloor M, Groenendijk P, Clerici S, Leng M, Jones C (2022) Paired analysis of tree ring width and carbon isotopes indicate when controls on tropical tree growth change from light to water limitations. Tree Physiol. https://doi.org/10.1093/treephys/tpab142
Brodribb TJ, Cochard H (2009) Hydraulic failure defines the recovery and point of death in water-stressed conifers. Plant Physiol 149:575–584
Brum M, Vadeboncoeur MA, Ivanov V, Asbjornsen H, Saleska S, Alves LF, Penha D, Dias JD, Aragao L, Barros F, Bittencourt P, Pereira L, Oliveira RS (2019) Hydrological niche segregation defines forest structure and drought tolerance strategies in a seasonal Amazon forest. J Ecol 107:318–333
Brunner I, Herzog C, Dawes MA, Arend M, Sperisen C (2015) How tree roots respond to drought. Front Plant Sci 6:547
Christensen-Dalsgaard KK, Fournier M, Ennos AR, Barfod AS (2007) Changes in vessel anatomy in response to mechanical loading in six species of tropical trees. New Phytol 176:610–622
Cintron B (1990) Cedrela odorata. Agriculture handbook. USDA, Washington, DC
Corona-Nunez RO, Campo J, Williams M (2018) Aboveground carbon storage in tropical dry forest plots in Oaxaca, Mexico. For Ecol Manag 409:202–214
Dawson TE, Pate JS (1996) Seasonal water uptake and movement in root systems of Australian phraeatophytic plants of dimorphic root morphology: A stable isotope investigation. Oecologia 107:13–20
Echeverria A, Anfodillo T, Soriano D, Rosell JA, Olson ME (2019) Constant theoretical conductance via changes in vessel diameter and number with height growth in Moringa oleifera. J Exp Bot 70:5765–5772
Enquist BJ (2002) Universal scaling in tree and vascular plant allometry: toward a general quantitative theory linking plant form and function from cells to ecosystems. Tree Physiol 22:1045–1064
Enquist BJ (2003) Cope’s Rule and the evolution of long-distance transport in vascular plants: allometric scaling, biomass partitioning and optimization. Plant Cell Environ 26:151–161
Fajardo A, McIntire EJB, Olson ME (2019) When short stature is an asset in trees. Trends Ecol Evol 34:193–199
Fajardo A, Martinez-Perez C, Cervantes-Alcayde MA, Olson ME (2020) Stem length, not climate, controls vessel diameter in two trees species across a sharp precipitation gradient. New Phytol 225:2347–2355
Fan ZX, Sterck F, Zhang SB, Fu PL, Hao GY (2017) Tradeoff between stem hydraulic efficiency and mechanical strength affects leaf-stem allometry in 28 ficus tree species. Front Plant Sci 8:1619
Finch KN, Jones FA, Cronn RC (2019) Genomic resources for the Neotropical tree genus Cedrela (Meliaceae) and its relatives. Bmc Genom 20:58
Garcia-Cervigon AI, Olano JM, von Arx G, Fajardo A (2018) Xylem adjusts to maintain efficiency across a steep precipitation gradient in two coexisting generalist species. Ann Bot 122:461–472
Gleason SM, Westoby M, Jansen S, Choat B, Hacke UG, Pratt RB, Bhaskar R, Brodribb TJ, Bucci SJ, Cao KF, Cochard H, Delzon S, Domec JC, Fan ZX, Feild TS, Jacobsen AL, Johnson DM, Lens F, Maherali H, Martinez-Vilalta J, Mayr S, McCulloh KA, Mencuccini M, Mitchell PJ, Morris H, Nardini A, Pittermann J, Plavcova L, Schreiber SG, Sperry JS, Wright IJ, Zanne AE (2016) Weak tradeoff between xylem safety and xylem-specific hydraulic efficiency across the world’s woody plant species. New Phytol 209:123–136
Guan X, Werner J, Cao K-F, Pereira L, Kaack L, McAdam SAM, Jansen S (2022) Stem and leaf xylem of angiosperm trees experiences minimal embolism in temperate forests during two consecutive summers with moderate drought. Plant Biol. https://doi.org/10.1111/plb.13384
Gutierrez-Vazquez BN, Cornejo-Oviedo EH, Gutierrez-Vazquez MH, Gomez-Cardenas M (2012) Variation and prediction of basic wood density in Cedrela odorata L. Rev Fitotec Mex 35:87–90
Hacke UG, Spicer R, Schreiber SG, Plavcova L (2017) An ecophysiological and developmental perspective on variation in vessel diameter. Plant Cell Environ 40:831–845
Hammond WM, Yu KL, Wilson LA, Will RE, Anderegg WRL, Adams HD (2019) Dead or dying? Quantifying the point of no return from hydraulic failure in drought-induced tree mortality. New Phytol 223:1834–1843
Hoeber S, Leuschner C, Kohler L, Arias-Aguilar D, Schuldt B (2014) The importance of hydraulic conductivity and wood density to growth performance in eight tree species from a tropical semi-dry climate. For Ecol Manag 330:126–136
Islam M, Rahman M, Bräuning A (2019) Impact of extreme drought on tree-ring width and vessel anatomical features of Chukrasia tabularis. Dendrochronologia 53:63–72. https://doi.org/10.1016/j.dendro.2018.11.007
Johnson D, Eckart P, Alsamadisi N, Noble H, Martin C (2018a) Polar auxin transport is implicated in vessel differentiation and spatial patterning during secondary growth in Populus. Am J Bot 105:1609–1609
Johnson DJ, Needham J, Xu CG, Massoud EC, Davies SJ, Anderson-Teixeira KJ, Bunyavejchewin S, Chambers JQ, Chang-Yang CH, Chiang JM, Chuyong GB, Condit R, Cordell S, Fletcher C, Giardina CP, Giambelluca TW, Gunatilleke N, Gunatilleke S, Hsieh CF, Hubbell S, Inman-Narahari F, Kassim AR, Katabuchi M, Kenfack D, Litton CM, Lum S, Mohamad M, Nasardin M, Ong PS, Ostertag R, Sack L, Swenson NG, Sun IF, Tan S, Thomas DW, Thompson J, Umana MN, Uriarte M, Valencia R, Yap S, Zimmerman J, McDowell NG, McMahon SM (2018b) Climate sensitive size-dependent survival in tropical trees. Nat Ecol Evol 2:1436–1442
Jones HG (1998) Stomatal control of photosynthesis and transpiration. J Exp Bot 49:387–398
Kassambara A (2019) rstatix: Pipe-Friendly Framework for Basic Statistical Tests
Klein T, Randin C, Korner C (2015) Water availability predicts forest canopy height at the globalscale. Ecol Lett 18:1311–1320
Knipfer T, Brodersen CR, Zedan A, Kluepfel DA, McElrone AJ (2015) Patterns of drought-induced embolism formation and spread in living walnut saplings visualized using X-ray microtomography. Tree Physiol 35:744–755
Korver RA, Koevoets IT, Testerink C (2018) Out of shape during stress: a key role for Auxin. Trends Plant Sci 23:783–793
Kotowska MM, Thom R, Zhang Y, Schenk HJ, Jansen S (2020) Within-tree variability and sample storage effects of bordered pit membranes in xylem of Acer pseudoplatanus. Trees 34:61–71. https://doi.org/10.1007/s00468-019-01897-4
Lamb AFA (1968) Cedrela odorata. Commonwealth Forestry Institute, University of Oxford, Oxford
Lechthaler S, Turnbull TL, Gelmini Y, Pirotti F, Anfodillo T, Adams MA, Petit G (2019) A standardization method to disentangle environmental information from axial trends of xylem anatomical traits. Tree Physiol 39:495–502
Lens F, Luteyn JL, Smets E, Jansen S (2004) Ecological trends in the wood anatomy of Vaccinioideae (Ericaceae s.l.). Flora 199:309–319
Lens F, Vos RA, Charrier G, van der Niet T, Merckx V, Baas P, Gutierrez JA, Jacobs B, Doria LC, Smets E, Delzon S, Janssens SB (2016) Scalariform-to-simple transition in vessel perforation plates triggered by differences in climate during the evolution of Adoxaceae. Ann Bot 118:1043–1056
Lenth R (2018) emmeans: Estimated Marginal Means, aka Least-Squares Means. R package version 1.3.0. https://CRAN.R-project.org/package=emmeans
Levionnois S, Jansen S, Wandji RT, Beauchêne J, Ziegler C, Coste S, Stahl C, Delzon S, Authier L, Heuret P (2021) Linking drought-induced xylem embolism resistance to wood anatomical traits in Neotropical trees. New Phytol 229:1453–1466
Liang X, He P, Liu H, Zhu S, Uyehara IK, Hou H, Wu G, Zhang H, You Z, Xiao Y, Ye Q (2019) Precipitation has dominant influences on the variation of plant hydraulics of the native Castanopsis fargesii (Fagaceae) in subtropical China. Agric for Meteorol 271:83–91. https://doi.org/10.1016/j.agrformet.2019.02.043
Liu H, Gleason SM, Hao G, Hua L, He P, Goldstein G, Ye Q (2019) Hydraulic traits are coordinated with maximum plant height at the global scale. Sci Adv 5:1332. https://doi.org/10.1126/sciadv.aav1332
Liu H, Ye Q, Gleason SM, He PC, Yin DY (2020) Weak tradeoff between xylem hydraulic efficiency and safety: climatic seasonality matters. New Phytol 229:1440–1452
Lobo A, Torres-Ruiz JM, Burlett R, Lemaire C, Parise C, Francioni C, Truffaut L, Tomaskova I, Hansen JK, Kjaer ED, Kremer A, Delzon S (2018) Assessing inter- and intraspecific variability of xylem vulnerability to embolism in oaks. For Ecol Manag 424:53–61
Locosselli GM, Buckeridge MS, Moreira MZ, Ceccantini G (2013) A multi-proxy dendroecological analysis of two tropical species (Hymenaea spp., Leguminosae) growing in a vegetation mosaic. Trees-Struct Funct 27:25–36
Locosselli GM, Brienen RJW, Leite MdS, Gloor M, Krottenthaler S, Oliveira AAd, Barichivich J, Anhuf D, Ceccantini G, Schöngart J, Buckeridge M (2020) Global tree-ring analysis reveals rapid decrease in tropical tree longevity with temperature. Proc Natl Acad Sci USA 117:33358
Losso A, Anfodillo T, Ganthaler A, Kofler W, Markl Y, Nardini A, Oberhuber W, Purin G, Mayr S (2018) Robustness of xylem properties in conifers: analyses of tracheid and pit dimensions along elevational transects. Tree Physiol 38:212–222
Manzoni S, Vico G, Thompson S, Beyer F, Weih M (2015) Contrasting leaf phenological strategies optimize carbon gain under droughts of different duration. Adv Water Resourc 84:37–51
Markesteijn L, Poorter L, Bongers F, Paz H, Sack L (2011) Hydraulics and life history of tropical dry forest tree species: coordination of species’ drought and shade tolerance. New Phytol 191:480–495
McDowell N, Pockman WT, Allen CD, Breshears DD, Cobb N, Kolb T, Plaut J, Sperry J, West A, Williams DG, Yepez EA (2008) Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New Phytol 178:719–739
Medeiros JS, Lens F, Maherali H, Jansen S (2019) Vestured pits and scalariform perforation plate morphology modify the relationships between angiosperm vessel diameter, climate and maximum plant height. New Phytol 221:1802–1813
Moles AT, Warton DI, Warman L, Swenson NG, Laffan SW, Zanne AE, Pitman A, Hemmings FA, Leishman MR (2009) Global patterns in plant height. J Ecol 97:923–932
Muellner AN, Pennington TD, Chase MW (2009) Molecular phylogenetics of Neotropical Cedreleae (mahogany family, Meliaceae) based on nuclear and plastid DNA sequences reveal multiple origins of “Cedrela odorata.” Mol Phylogenet Evol 52:461–469
Muellner AN, Pennington TD, Koecke AV, Renner SS (2010) Biogeography of Cedrela (Meliaceae, Sapindales) in Central and South America. Am J Bot 97:511–518
Nakagawa S, Schielzeth H (2013) A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol Evol 4:133–142
Olson ME, Anfodillo T, Rosell JA, Petit G, Crivellaro A, Isnard S, León-Gómez C, Alvarado-Cárdenas LO, Castorena M (2014) Universal hydraulics of the flowering plants: vessel diameter scales with stem length across angiosperm lineages, habits and climates. Ecol Lett 17:988–997
Olson ME, Soriano D, Rosell JA, Anfodillo T, Donoghue MJ, Edwards EJ, Leon-Gomez C, Dawson T, Martinez JJC, Castorena M, Echeverria A, Espinosa CI, Fajardo A, Gazol A, Isnard S, Lima RS, Marcati CR, Mendez-Alonzo R (2018) Plant height and hydraulic vulnerability to drought and cold. Proc Natl Acad Sci USA 115:7551–7556
Perez-Garcia EA, Meave JA, Villasenor JL, Gallardo-Cruz JA, Lebrija-Trejos EE (2010) Vegetation heterogeneity and life-strategy diversity in the flora of the heterogeneous landscape of Nizanda, Oaxaca, Mexico. Folia Geobot 45:143–161
Peterson TC, Vose RS (1997) Global Historical Climatology Network - Monthly (GHCN-M), Version 3. GHCN-M (all) precipitation. NOAA National Centers for Environmental Information
Petit G, Pfautsch S, Anfodillo T, Adams MA (2010) The challenge of tree height in Eucalyptus regnans: when xylem tapering overcomes hydraulic resistance. New Phytol 187:1146–1153
Pfautsch S, Harbusch M, Wesolowski A, Smith R, Macfarlane C, Tjoelker MG, Reich PB, Adams MA (2016) Climate determines vascular traits in the ecologically diverse genus Eucalyptus. Ecol Lett 19:240–248
Pittermann J, Choat B, Jansen S, Stuart SA, Lynn L, Dawson TE (2010) The relationships between xylem safety and hydraulic efficiency in the cupressaceae: the evolution of pit membrane form and function. Plant Physiol 153:1919–1931
Prendin AL, Mayr S, Beikircher B, von Arx G, Petit G (2018) Xylem anatomical adjustments prioritize hydraulic efficiency over safety as Norway spruce trees grow taller. Tree Physiol 38:1088–1097
Pumijumnong N, Park WK (1999) Vessel chronologies from teak in northern Thailand and their climatic signal. Iawa J 20:285–294
R Core Team (2018) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Ramesha MN, Link RM, Paligi SS, Hertel D, Röll A, Hölscher D, Schuldt B (2022) Variability in growth-determining hydraulic wood and leaf traits in Melia dubia across a steep water availability gradient in southern India. For Ecol Manag 505:119875. https://doi.org/10.1016/j.foreco.2021.119875
Rosell JA, Olson ME, Anfodillo T (2017) Scaling of xylem vessel diameter with plant size: causes, predictions, and outstanding questions. Curr for Reports 3:46–59
Rowland L, da Costa ACL, Galbraith DR, Oliveira RS, Binks OJ, Oliveira AAR, Pullen AM, Doughty CE, Metcalfe DB, Vasconcelos SS, Ferreira LV, Malhi Y, Grace J, Mencuccini M, Meir P (2015) Death from drought in tropical forests is triggered by hydraulics not carbon starvation. Nature 528:119
Ryan MG, Yoder BJ (1997) Hydraulic limits to tree height and tree growth. Bioscience 47:235–242
Savage VM, Bentley LP, Enquist BJ, Sperry JS, Smith DD, Reich PB, von Allmen EI (2010) Hydraulic trade-offs and space filling enable better predictions of vascular structure and function in plants. Proc Natl Acad Sci USA 107:22722–22727
Scheffer M, Xu C, Hantson S, Holmgren M, Los SO, van Nes EH (2018) A global climate niche for giant trees. Global Change Biol 24:2875–2883
Scholz A, Klepsch M, Karimi Z, Jansen S (2013) How to quantify conduits in wood? Front Plant Sci 4:56
Schwendenmann L, Pendall E, Sanchez-Bragado R, Kunert N, Holscher D (2015) Tree water uptake in a tropical plantation varying in tree diversity: interspecific differences, seasonal shifts and complementarity. Ecohydrology 8:1–12
Scoffoni C, Albuquerque C, Brodersen CR, Townes SV, John GP, Cochard H, Buckley TN, McElrone AJ, Sack L (2017) Leaf vein xylem conduit diameter influences susceptibility to embolism and hydraulic decline. New Phytol 213:1076–1092
Shenkin A, Bolker B, Pena-Claros M, Licona JC, Ascarrunz N, Putz FE (2018) Interactive effects of tree size, crown exposure and logging on drought-induced mortality. Philos Trans R Soc B Biol Sci. https://doi.org/10.1098/rstb.2018.0189
Sperry JS (1986) Relationship of xylem embolism to xylem pressure potential, stomatal closure, and shoot morphology in the palm rhapis-excelsa. Plant Physiol 80:110–116
Sperry JS, Love DM (2015) What plant hydraulics can tell us about responses to climate-change droughts. New Phytol 207:14–27
Sperry JS, Tyree MT (1988) Mechanism of water stress-induced xylem embolism. Plant Physiol 88:581–587
Sperry JS, Hacke UG, Pittermann J (2006) Size and function in conifer tracheids and angiosperm vessels. Am J Bot 93:1490–1500
Stovall AEL, Shugart H, Yang X (2019) Tree height explains mortality risk during an intense drought. Nat Commun 10:4385
Tao SL, Guo QH, Li C, Wang ZH, Fang JY (2016) Global patterns and determinants of forest canopy height. Ecology 97:3265–3270
Tardieu F, Simonneau T (1998) Variability among species of stomatal control under fluctuating soil water status and evaporative demand: modelling isohydric and anisohydric behaviours. J Exp Bot 49:419–432
Tyree MT, Ewers FW (1991) The hydraulic architecture of trees and other woody-plants. New Phytol 119:345–360
Tyree MT, Sperry JS (1989) Vulnerability of xylem to cavitation and embolism. Ann Rev Plant Physiol Plant Mol Biol 40:19–38
Valencia R, Condit R, Foster RB, Romoleroux K, Villa G, Svenning J-C, Magård E, Bass M, Losos EC, Balslev H (2004) Yasuní forest dynamics plot, Ecuador. In: Losos EC, Leigh ECJ (eds) Tropical forest diversity and dynamism: findings from a large-scale plot network. The University of Chicago Press, Chicago, pp 609–620
Vico G, Dralle D, Feng X, Thompson S, Manzoni S (2017) How competitive is drought deciduousness in tropical forests? A combined eco-hydrological and eco-evolutionary approach. Environ Res Lett 12:065006
Vilagrosa A, Chirino E, Peguero-Pina J, Barigah T, Cochard H, Gil-Pelegrín E (2012) Xylem cavitation and embolism in plants living in water-limited ecosystems. In: Aroca R (ed) Plant responses to drought stress. Springer, Berlin, pp 63–109
Villagra M, Campanello PI, Bucci SJ, Goldstein G (2013a) Functional relationships between leaf hydraulics and leaf economic traits in response to nutrient addition in subtropical tree species. Tree Physiol 33:1308–1318
Villagra M, Campanello PI, Montti L, Goldstein G (2013b) Removal of nutrient limitations in forest gaps enhances growth rate and resistance to cavitation in subtropical canopy tree species differing in shade tolerance. Tree Physiol 33:285–296
Warwick NWM, Hailey L, Clarke KL, Gasson PE (2017) Climate trends in the wood anatomy of Acacia sensu stricto (Leguminosae: Mimosoideae). Ann Bot 119:1249–1266
Wason JW, Anstreicher KS, Stephansky N, Huggett BA, Brodersen CR (2018) Hydraulic safety margins and air-seeding thresholds in roots, trunks, branches and petioles of four northern hardwood trees. New Phytol 219:77–88. https://doi.org/10.1111/nph.15135
West GB, Brown JH, Enquist BJ (1999) A general model for the structure and allometry of plant vascular systems. Nature 400:664–667
White DA, Hood CS (2004) Vegetation patterns and environmental gradients in tropical dry forests of the northern Yucatan Peninsula. J Veg Sci 15:151–160
Woodruff DR, Meinzer FC (2011) Size-dependent changes in biophysical control of tree growth: the role of turgor. In: Meinzer F, Lachenbruch B, Dawson T (eds) Size- and age-related changes in tree structure and function. Springer, Dordrecht
Worbes M (1999) Annual growth rings, rainfall-dependent growth and long-term growth patterns of tropical trees from the Caparo Forest Reserve in Venezuela. J Ecol 87:391–403
Zweifel R, Zimmermann L, Zeugin F, Newbery DM (2006) Intra-annual radial growth and water relations of trees: implications towards a growth mechanism. J Exp Bot 57:1445–1459
Acknowledgements
The authors acknowledge the help of Dr Santiago Clerici who aided the preparation of vessel images and the Centro de Invetigaciones en Ecosistemas from the UNAM in Morelia and the people of Nizanda for the warm reception and help during field and lab work.
Funding
A C-O was funded by the Leeds-York NERC DTP (NE/L002574/1). RJWB. was supported through Dirección General de Asuntos del Personal Académico of the UNAM (Mexico), UK-NERC Research fellowship (NE/L0211160/1), and through UK-NERC research grant NE/S008659/1, E.G. was supported by UK-NERC grant NE/N012542/1. DG acknowledges support from two NERC-funded research grants: TREMOR (NE/N004655/1) and ARBOLES (NE/S011811/1). PG received financial support from the Miquelfonds and Alberta Mennega Stichting to realise fieldwork and acknowledges current funding by the São Paulo Research Foundation (FAPESP grant 2018/01847‐0). This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by ACO, RB, and PG. The first draft of the manuscript was written by ACO and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chambers-Ostler, A., Gloor, E., Galbraith, D. et al. Vessel tapering is conserved along a precipitation gradient in tropical trees of the genus Cedrela. Trees 37, 269–284 (2023). https://doi.org/10.1007/s00468-022-02345-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-022-02345-6