Abstract
Key message
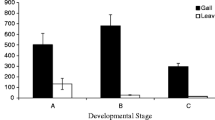
Massive infection of Populus × petrovskiana leaves by petiole gall aphids (Pemphigus spyrothecae) significantly decreased leaf dry mass per unit area, N content per dry mass and net assimilation rate per area, and increased stomatal conductance, leaf dry mass per fresh mass, and constitutive emissions of isoprene. The infection also induced emissions of green leaf volatiles, monoterpenes and benzenoids. The emissions scaled with the infection severity as assessed by dry gall mass per leaf dry mass.
Abstract
Poplar spiral gall aphid (Pemphigus spyrothecae) forms galls on the petiole in poplars (Populus) and mass infestations are frequent in poplar stands, but how these parasite gall infestations can affect the leaf lamina structure, photosynthetic rate and constitutive and stress volatile emissions is unknown. We investigated how the infestation by the petiole gall aphids affects lamina photosynthetic characteristics (net assimilation rate, stomatal conductance), C and N contents, and constitutive isoprene and induced volatile emissions in Populus × petrovskiana. The dry gall mass per leaf dry mass (Mg/Ml) was used as a quantitative measure of the severity of gall infestation. Very high fraction of leaf biomass was invested in gall formation with Mg/Ml varying between 0.5 and 2. Over the whole range of the infestation severities, net assimilation rate per area, leaf dry mass per unit area and N content decreased with increasing the severity of infestation. In contrast, stomatal conductance, leaf dry mass per fresh mass, constitutive isoprene emissions, and induced green leaf volatile (GLV), monoterpene, sesquiterpene and benzenoid emissions increased with increasing the severity of gall infestation. The rates of induced emissions were low and these emissions were associated with methyl jasmonate release from leaf laminas. The data demonstrate that petiole gall infestations lead to major changes in leaf lamina sink–source relationships and leaf water relations, thereby significantly altering lamina photosynthesis. Modifications in stress-induced emissions likely indicated systemic signaling triggered by jasmonate transported from the petiole galls to the lamina where jasmonate elicited a cascade of volatile emission responses. Enhanced isoprene emissions and induced volatile emissions can play a major role in indirect defense against other herbivores, securing the food source for the gall aphids. In conclusion, a massive infestation by petiole gall aphids can profoundly modify the foliage photosynthetic performance and volatile emission profiles in poplars.







Similar content being viewed by others
References
Aasamaa K, Sõber A, Hartung W, Niinemets Ü (2002) Rate of stomatal opening, shoot hydraulic conductance and photosynthesis characteristics in relation to leaf abscisic acid concentration in six temperate deciduous trees. Tree Physiol 22:267–276
Ameye M, Allmann S, Verwaeren J, Smagghe G, Haesaert G, Schuurink RC, Audenaert K (2017) Green leaf volatile production by plants: a meta-analysis. New Phytol. https://doi.org/10.1111/nph.14671
Arimura GI, Ozawa R, Shimoda T, Nishioka T, Boland W, Takabayashi J (2000) Herbivory-induced volatiles elicit defence genes in lima bean leaves. Nature 406:512–515
Arimura GI, Ozawa R, Horiuchi J, Nishioka T, Takabayashi J (2001) Plant-plant interactions mediated by volatiles emitted from plants infested by spider mites. Biochem Syst Ecol 29:1049–1061
Arimura GI, Huber DPW, Bohlmann J (2004) Forest tent caterpillars (Malacosoma disstria) induce local and systemic diurnal emissions of terpenoid volatiles in hybrid poplar (Populus trichocarpa × deltoides): cDNA cloning, functional characterization, and patterns of gene expression of (–)-germacrene D synthase, PtdTPS1. Plant J 37:603–616
Beauchamp J, Wisthaler A, Hansel A, Kleist E, Miebach M, Niinemets Ü, Schurr U, Wildt J (2005) Ozone induced emissions of biogenic VOC from tobacco: relationships between ozone uptake and emission of LOX products. Plant Cell Environ 28:1334–1343
Besten MA, Nunes DS, Granato D, Sens SL, Wisniewski A Jr, Simionatto EL, Riva-Scharf D (2015) Volatile components from galls induced by Baccharopelma dracunculifoliae (Hemiptera: Psyllidae) on leaves of Baccharis dracunculifolia (Asteraceae). Quim Nova 38:66–70
Blackman RL, Eastop VF (2001) Aphids on the world’s trees: an identification and information guide. Orient Insects 35:104
Blande JD, Tiiva P, Oksanen E, Holopainen JK (2007) Emission of herbivore-induced volatile terpenoids from two hybrid aspen (Populus tremula × tremuloides) clones under ambient and elevated ozone concentrations in the field. Glob Change Biol 13:2538–2550
Brilli F, Ruuskanen TM, Schnitzhofer R, Müller M, Breitenlechner M, Bittner V, Wohlfahrt G, Loreto F, Hansel A (2011) Detection of plant volatiles after leaf wounding and darkening by proton transfer reaction “time-of-flight” mass spectrometry (PTR-TOF). PLoS One 6:e20419
Caemmerer SV, Farquhar GD (1981) Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153:376–387
Chapman RF (2013) Chap. 2: mouthparts and feeding. In: The insects: structure and function. Cambridge University Press, Cambridge, p 961
Cheong JJ, Choi YD (2003) Methyl jasmonate as a vital substance in plants. Trends Genet 19:409–413
Compson ZG, Larson KC, Zinkgraf MS, Whitham TG (2011) A genetic basis for the manipulation of sink-source relationships by the galling aphid Pemphigus betae. Oecologia 167:711–721
Comstock JP (2002) Hydraulic and chemical signalling in the control of stomatal conductance and transpiration. J Exp Bot 53:195–200
Copolovici L, Niinemets Ü (2010) Flooding induced emissions of volatile signaling compounds in three tree species with differing waterlogging tolerance. Plant Cell Environ 33:1582–1594
Copolovici L, Niinemets Ü (2016) Environmental impacts on plant volatile emission. In: Blande J, Glinwood R (eds) Deciphering chemical language of plant communication. Springer International Publishing, Berlin, pp 35–59
Copolovici L, Kännaste A, Niinemets Ü (2009) Gas chromatography-mass spectrometry method for determination of monoterpene and sesquiterpene emissions from stressed plants. Stud Univ Babes-Bolyai Chem 54:329–339
Copolovici L, Väärtnõu F, Portillo Estrada M, Niinemets Ü (2014) Oak powdery mildew (Erysiphe alphitoides)-induced volatile emissions scale with the degree of infection in Quercus robur. Tree Physiol 34:1399–1410
Copolovici L, Pag A, Kännaste A, Bodescu A, Tomescu D, Copolovici D, Soran ML, Niinemets Ü (2017) Disproportionate photosynthetic decline and inverse relationship between constitutive and induced volatile emissions upon feeding of Quercus robur leaves by large larvae of gypsy moth (Lymantria dispar). Environ Exp Bot 138:184–192
Davies PJ (2004) The plant hormones: their nature, occurrence, and functions. In: Davies PJ (ed) Plant hormones: biosynthesis, signal transduction, action! Academic Publishers, Dordrecht, pp 1–15
De Moraes CM, Lewis WJ, Pare PW, Alborn HT, Tumlinson JH (1998) Herbivore-infested plants selectively attract parasitoids. Nature 393:570–573
De Moraes CM, Mescher MC, Tumlinson JH (2001) Caterpillar-induced nocturnal plant volatiles repel nonspecific females. Nature 410:577–580
Dorchin N, Cramer MD, Hoffmann JH (2006) Photosynthesis and sink activity of wasp-induced galls in Acacia pycnantha. Ecology 87:1781–1791
Erb M, Meldau S, Howe GA (2012) Role of phytohormones in insect-specific plant reactions. Trends Plant Sci 17:250–259
Fäldt J, Arimura GI, Gershenzon J, Takabayashi J, Böhlmann J (2003) Functional identification of AtTPS03 as (E)-ß-ocimene synthase: a monoterpene synthase catalyzing jasmonate- and wound-induced volatile formation in Arabidopsis thaliana. Planta 216:745–751
Flexas J, Barbour MM, Brendel O, Cabrera HM, Carriquí M, Díaz-Espejo A, Douthe C, Dreyer E, Ferrio JP, Gago J, Gallé A, Galmés J, Kodama N, Medrano H, Niinemets Ü, Peguero-Pina JJ, Pou A, Ribas-Carbó M, Tomás M, Tosens T, Warren CR (2012) Mesophyll diffusion conductance to CO2: an unappreciated central player in photosynthesis. Plant Sci 193–194:70–84
Frost CJ, Mescher MC, Dervinis C, Davis JM, Carlson JE, De Moraes CM (2008) Priming defense genes and metabolites in hybrid poplar by the green leaf volatile cis-3-hexenyl acetate. New Phytol 180:722–734
Giron D, Huguet E, Stone GN, Body M (2016) Insect-induced effects on plants and possible effectors used by galling and leaf-mining insects to manipulate their host-plant. J Insect Physiol 84:70–89
Grote R, Monson RK, Niinemets Ü (2013) Leaf-level models of constitutive and stress-driven volatile organic compound emissions. In: Niinemets Ü, Monson RK (eds) Biology, controls and models of tree volatile organic compound emissions. Springer, Berlin, pp 315–355
Hałaj R, Osiadacz B (2013) European gall-forming Pemphigus (Aphidoidea:Eriosomatidae). Zool Anz 252:417–423
Hall CR, Carroll AR, Kitching RL (2017) A meta-analysis of the effects of galling insects on host plant secondary metabolites. Arthropod Plant Interact 11:463–473
Heil M, Ton J (2008) Long-distance signaling in plant defense. Trends Plant Sci 13:264–272
Holopainen JK, Nerg AM, Blande JD (2013) Multitrophic signalling in polluted atmospheres. In: Niinemets Ü, Monson RK (eds) Biology, controls and models of tree volatile organic compound emissions. Springer, Berlin, pp 285–314
Howe GA, Jander G (2008) Plant Immunity to Insect Herbivores. Annu Rev Plant Biol 59(1):41
Irmisch S, Jiang YF, Chen F, Gershenzon J, Köllner GT (2014) Terpene synthases and their contribution to herbivore-induced volatile emission in western balsam poplar (Populus trichocarpa). BMC Plant Biol 14:1–16
Jeschke WD, Baig A, Hilpert A (1997) Sink-stimulated photosynthesis, increased transpiration and increased demand-dependent stimulation of nitrate uptake: nitrogen and carbon relations in the parasitic association Cuscuta reflexa-Coleus blumei. J Exp Bot 48:915–925
Jiang YF, Ye JY, Veromann L-L, Niinemets Ü (2016) Scaling of photosynthesis and constitutive and induced volatile emissions with severity of leaf infection by rust fungus (Melampsora larici-populina) in Populus balsamifera var. suaveolens. Tree Physiol 36(7):856–872
Jiang YF, Veromann L-L, Ye JY, Niinemets Ü (2017a) Oak gall wasp infections of Quercus robur leaves lead to profound modifications in foliage photosynthetic and volatile emission characteristics. Plant Cell Environ 41(1):160–175
Jiang YF, Ye JY, Li S, Niinemets Ü (2017b) Methyl jasmonate-induced emission of biogenic volatiles is biphasic in cucumber: a high-resolution analysis of dose dependence. J Exp Bot 68:4679–4694
Jordi W, Schapendonk A, Davelaar E, Stoopen GM, Pot CS, De Visser R, Van Rhijn JA, Gan S, Amasino RM (2000) Increased cytokinin levels in transgenic P-SAG12-IPT tobacco plants have large direct and indirect effects on leaf senescence, photosynthesis and N partitioning. Plant Cell Environ 23:279–289
Kännaste A, Copolovici L, Niinemets Ü (2014) Gas chromatography mass-spectrometry method for determination of biogenic volatile organic compounds emitted by plants. In: Rodríguez-Concepción M (ed) Plant isoprenoids: methods and protocols. Humana Press, New York, pp 161–169
Künkler N, Brandl R, Brändle M (2013) Changes in clonal poplar leaf chemistry caused by stem galls alter herbivory and leaf litter decomposition. PLOS One 8:e79994. https://doi.org/10.1371/journal.pone.0079994
Kurzfeld-Zexer L, Wool D, Inbar M (2010) Modification of tree architecture by a gall-forming aphid. Trees-Struct Funct 24:13–18
Larson KC, Whitham TG (1991) Manipulation of food resources by a gall-forming aphid: the physiology of sink-source interactions. Oecologia 88:15–21
Lavigne MB, Little CHA, Major JE (2001) Increasing the sink: source balance enhances photosynthetic rate of 1-year-old balsam fir foliage by increasing allocation of mineral nutrients. Tree Physiol 21:417–426
Li T, Blande JD (2017) Volatile-mediated within-plant signaling in hybrid aspen: required for systemic responses. J Chem Ecol 43(4):1–12
Loreto F, Sharkey TD (1990) A gas-exchange study of photosynthesis and isoprene emission in Quercus rubra L. Planta 182:523–531
McCormick AC, Boeckler GA, Köllner TG, Gershenzon J, Unsicker SB (2014) The timing of herbivore-induced volatile emission in black poplar (Populus nigra) and the influence of herbivore age and identity affect the value of individual volatiles as cues for herbivore enemies. BMC Plant Biol 14:304
Monson RK, Grote R, Niinemets Ü, Schnitzler JP (2012) Tansley review. Modeling the isoprene emission rate from leaves. New Phytol 195:541–559
Müller M, Graus M, Ruuskanen TM, Schnitzhofer R, Bamberger I, Kaser L, Titzmann T, Hörtnagl L, Wohlfahrt G, Karl T, Hansel A (2010) First eddy covariance flux measurements by PTR-TOF. Atmos Meas Tech 3:387–395
Niinemets Ü (1999) Components of leaf dry mass per area-thickness and density-alter leaf photosynthetic capacity in reverse directions in woody plants. New Phytol 144:35–47
Niinemets Ü (2001) Global-scale climatic controls of leaf dry mass per area, density, and thickness in trees and shrubs. Ecology 82:453–469
Niinemets Ü, Kull O, Tenhunen JD (2004) Within canopy variation in the rate of development of photosynthetic capacity is proportional to integrated quantum flux density in temperate deciduous trees. Plant Cell Environ 27:293–313
Niinemets Ü, Kuhn U, Harley PC, Staudt M, Arneth A, Cescatti A, Ciccioli P, Copolovici L, Geron C, Guenther A, Kesselmeier J, Lerdau MT, Monson RK, Peñuelas J (2011) Estimations of isoprenoid emission capacity from enclosure studies: measurements, data processing, quality and standardized measurement protocols. Biogeosciences 8:2209–2246
Niinemets Ü, García-Plazaola JI, Tosens T (2012) Photosynthesis during leaf development and ageing. In: Flexas J, Loreto F, Medrano H (eds) Terrestrial photosynthesis in a changing environment. A molecular, physiological and ecological approach. Cambridge University Press, Cambridge, pp 353–372
Niinemets Ü, Kännaste A, Copolovici L (2013) Quantitative patterns between plant volatile emissions induced by biotic stresses and the degree of damage. Front Plant Sci 4:262
Onoda Y, Wright IJ, Evans JR, Hikosaka K, Kitajima K, Niinemets Ü, Poorter H, Tosens T, Westoby M (2017) Physiological and structural tradeoffs underlying the leaf economics spectrum. New Phytol 214:1447–1463
Osiadacz B, Hałaj R (2009) The aphids (Hemiptera: Sternorrhyncha: Aphidinea) of Poland. A distributional checklist. Polish Entomol Monogr 6:1–96
Patankar R, Thomas SC, Smith SM (2011) A gall-inducing arthropod drives declines in canopy tree photosynthesis. Oecologia 167:701–709
Pazouki L, Memari HR, Kännaste A, Bichele R, Niinemets Ü (2015) Germacrene A synthase in yarrow (Achillea millefolium) is an enzyme with mixed substrate specificity: gene cloning, functional characterization and expression analysis. Front Plant Sci 6:111
Portillo-Estrada M, Niinemets Ü (2018) Massive release of volatile organic compounds due to leaf midrib wounding in Populus tremula. Plant Ecol 219(9):1021–1028
Portillo-Estrada M, Kazantsev T, Talts E, Tosens T, Niinemets Ü (2015) Emission timetable and quantitative patterns of wound-induced volatiles across different leaf damage treatments in aspen (Populus tremula). J Chem Ecol 41:1105–1117
Possell M, Loreto F (2013) The role of volatile organic compounds in plant resistance to abiotic stresses: responses and mechanisms. In: Niinemets Ü, Monson RK (eds) Biology, controls and models of tree volatile organic compound emissions. Springer, Berlin, pp 209–235
Rand K, Bar E, Ben Ari M, Davidovich-Rikanati R, Dudareva N, Inbar M, Lewinsohn E (2017) Differences in monoterpene biosynthesis and accumulation in Pistacia palaestina leaves and aphid-induced galls. J Chem Ecol 43:143–152
Rasulov B, Hüve K, Välbe M, Laisk A, Niinemets Ü (2009) Evidence that light, carbon dioxide and oxygen dependencies of leaf isoprene emission are driven by energy status in hybrid aspen. Plant Physiol 151:448–460
Rasulov B, Bichele I, Laisk A, Niinemets Ü (2014) Competition between isoprene emission and pigment synthesis during leaf development in aspen. Plant Cell Environ 37:724–741
Redfern M (2011) Plant galls. The new naturalist library. Harper Collins Publishers, London, p 562
Richardson RA, Body M, Warmund MR, Schultz JC, Appel HM (2016) Morphometric analysis of young petiole galls on the narrow-leaf cottonwood, Populus angustifolia, by the sugarbeet root aphid, Pemphigus betae. Protoplasma 254:203–216
Roden JS, Pearcy RW (1993) Effect of leaf flutter on the light environment of poplars. Oecologia 93:201–207
Schoonhoven LM, Van Loon JJA, Dicke M (2005) Insect-plant biology. Oxford University Press, Oxford, p 440
Sharkey TD, Yeh S (2001) Isoprene emission from plants. Annu Rev Plant Biol 52:407–436
Shour M, Jesse L, Lewis D (2004) Insect galls on trees and shrubs. http://www.oakgov.com/msu/Documents/publication/ic417_insect_galls.pdf
Sun Z, Copolovici L, Niinemets Ü (2012) Can the capacity for isoprene emission acclimate to environmental modifications during autumn senescence in temperate deciduous tree species Populus tremula? J Plant Res 125:263–274
Takei M, Yoshida S, Kawai T, Hasegawa M, Suzuki Y (2015) Adaptive significance of gall formation for a gall-inducing aphids on Japanese elm trees. J Insect Physiol 72:43–51
Tamogami S, Rakwal R, Agrawal GK (2008) Interplant communication: airborne methyl jasmonate is essentially converted into JA and JA-Ile activating jasmonate signaling pathway and VOCs emission. Biochem Bioph Res Co 376:723–727
Tomás M, Flexas J, Copolovici L, Galmés J, Hallik L, Medrano H, Tosens T, Vislap V, Niinemets Ü (2013) Importance of leaf anatomy in determining mesophyll diffusion conductance to CO2 across species: quantitative limitations and scaling up by models. J Exp Bot 64:2269–2281
Tooker JF, Rohr JR, Abrahamson WG, De Moraes CM (2008) Gall insects can avoid and alter indirect plant defenses. New Phytol 178:657–671
Toome M, Randjärv P, Copolovici L, Niinemets Ü, Heinsoo K, Luik A, Noe SM (2010) Leaf rust induced volatile organic compounds signalling in willow during the infection. Planta 232:235–243
Tosens T, Nishida K, Gago J, Coopman R, Cabrera HM, Carriquí M, Laanisto L, Morales L, Nadal M, Rojas R, Talts E, Tomas M, Hanba YT, Niinemets Ü, Flexas J (2016) The photosynthetic capacity in 35 ferns and fern allies: mesophyll CO2 diffusion as a key trait. New Phytol 209:1576–1590
Vickers CE, Gershenzon J, Lerdau MT, Loreto F (2009) A unified mechanism of action for volatile isoprenoids in plant abiotic stress. Nat Chem Biol 5:283–291
Walling L (2000) The myriad of plant responses to herbivores. J Plant Growth Regul 19:195–216
Wasternack C, Hause B (2013) Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. Ann Bot 111:1021–1058
Wilkinson MJ, Monson RK, Trahan N, Lee S, Brown E, Jackson RB, Polley HW, Fay PA, Fall R (2009) Leaf isoprene emission rate as a function of atmospheric CO2 concentration. Glob Change Biol 15:1189–1200
Wool D (2004) Galling aphids: specialization, biological complexity, and variation. Annu Rev Entomol 49:175
Yli-Pirilä P, Copolovici L, Kännaste A, Noe S, Blande JD, Mikkonen S, Klemola T, Pulkkinen J, Virtanen A, Laaksonen A, Joutsensaari J, Niinemets Ü, Holopainen JK (2016) Herbivory by an outbreaking moth increases emissions of biogenic volatiles and leads to enhanced secondary organic aerosol formation capacity. Environ Sci Technol 50:11501–11510
Acknowledgements
This study has been funded by the European Research Council (Advanced Grant 322603, SIP-VOL+), the Estonian Ministry of Science and Education (Institutional Grant IUT-8-3) and the European Commission through the European Regional Fund (Center of Excellence EcolChange).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Koike.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
. Schematic overview of the two-channel custom-designed gas-exchange system applied for measurements of photosynthesis, transpiration and trace gas emissions in Populus × petrovskiana leaf infested by Pemphigus spyrothecae aphid galls. (DOCX 22 KB)
Rights and permissions
About this article
Cite this article
Ye, J., Jiang, Y., Veromann-Jürgenson, LL. et al. Petiole gall aphid (Pemphigus spyrothecae) infestation of Populus × petrovskiana leaves alters foliage photosynthetic characteristics and leads to enhanced emissions of both constitutive and stress-induced volatiles. Trees 33, 37–51 (2019). https://doi.org/10.1007/s00468-018-1756-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-018-1756-2