Abstract
Key message
Relationship between sap flow and functional traits changes with altitude and changes in water availability can impose a conservative water use in woody species of tropical rainforest.
Abstract
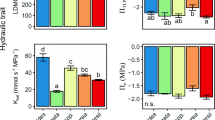
Using a trait-based approach, we have identified that tropical trees are vulnerable to decreases in water availability, especially in montane areas, where higher radiation and vapor pressure deficits lead to higher water loss from trees. Changes to functional traits are useful descriptors of the response of species to variation in resource availability and environmental conditions. However, how these trait-environment relationships change with altitude remains unclear. We investigated changes in xylem sap flow along an altitudinal variation and evaluated the contribution of morphological traits to total plant water use. We hypothesize that (1) at the Montane forest, plant species will show a more conservative water use and (2) seasonally, there will be a much greater increase in conservative water use during the dry season at the Lowland site, since the climate conditions in the Montane site impose constraints to water use throughout the year. Remarkably, although water is assumed to be a non-limiting resource for Atlantic rainforest in general, we observed ecophysiological adjustments for more conservative water use in Montane forest. Our findings demonstrate that changes to water supply and demand as determined by rainfall, VPD and soil water storage can impose restrictions to water loss which differ across spatio-temporal scales. We suggest that the next steps for research in Montane forest should focus on traits related to hydraulic failure and carbon starvation to address the question whether the higher conservative water use observed at the Montane Forest translates into a higher or lower susceptibility to intensification of drought which might arise due to climate change.






Similar content being viewed by others
References
Alves LF, Vieira SA, Scaranello MA, Camargo PB, Santos FAM, Joly CA, Martinelli LA (2010) Forest structure and live aboveground biomass variation along an elevational gradient of tropical Atlantic moist forest (Brazil). For Ecol Manage 260:679–691
Anderegg LDL, Anderegg WRL, Berry JA (2013) Not all droughts are created equal: translating meteorological drought into woody plant mortality. Tree Physiology 33:672–683
Andrade JL, Meinzer F, Goldstein G, Holbrook NM, Cavelier J, Jackson P, Silvera K (1998) Regulation of water flux through trunks, branches, and leaves in trees of a lowland tropical forest. Oecologia 115:463–471
Behling H (2008) Tropical mountain forest dynamics in Mata Atlantica and northern Andean biodiversity hotspots during the late Quaternary. In: Gradstein SR, Homeier J, Gansert D (eds) The tropical mountain forest patterns and processes in a biodiversity hotspot, vol 2. Universitätsverlag Göttingen, Göttingen, pp 25–34
Beier C, Beierkuhnlein C, Wohlgemuth T, Penuelas J, Emmett B, Körner C, de Boeck H, Christensen JH, Leuzinger S, Janssens IA, Hansen K (2012) Precipitation manipulation experiments—challenges and recommendations for the future. Ecol Lett 15:899–911
Bencke CSC, Morellato PC (2002) Estudo comparativo da fenologia de nove espécies arbóreas em três tipos de floresta atlântica no sudeste do Brasil. Rev Brasil de Bot 25:237–248
Bigelow S (2001) Evapotranspiration modelled from stands of three broad-leaved tropical trees in Costa Rica. Hydrol Process 15:2779–2796
Bruijnzeel LA, Veneklaas EJ (1998) Climatic conditions and tropical montane forest productivity: the fog has not lifted yet. Ecology 79:3–9
Bucci SJ, Goldstein G, Meinzer FC, Scholz FG, Franco AC, Bustamante M (2004) Functional convergence in hydraulic architecture and water relations of tropical savanna trees: from leaf to whole plant. Tree Physiol 24:891–899
Burgess SSO (2006) Measuring transpiration responses to summer precipitation in a Mediterranean climate: a simple screening tool for identifying plant water-use strategies. Physiol Plant 127:404–412
Burgess SSO, Dawson TE (2004) The contribution of fog to the water relations of Sequoia sempervirens (D. Don): foliar uptake and prevention of dehydration. Plant Cell Environ 27:1023–1034
Burgess SSO, Adams MA, Turner NC, Ong CK (1998) The redistribution of soil water by tree root systems. Oecologia 115:306–311
Burgess SSO, Adams MA, Turner NC, Beverly CR, Ong CK, Khan AAH, Bleby TM (2001) An improved heat pulse method to measure low and reverse rates of sap flow in woody plants. Tree Physiol 21:589–598
Cavelier J (1996) Environmental factors and ecophysiological process along altitudinal gradients in wet tropical mountains. In: Mulkey SS, Chazdon RL, Smith AP (eds) Tropical forest plant ecophysiology. Chapman & Hall, New York, pp 399–439
Cavender-Bares J, Sack L, Savage J (2007) Atmospheric and soil drought reduce nocturnal conductance in live oaks. Tree Physiol 27:611–620
Chave J, Coomes D, Jansen S, Lewis SL, Swenson NG, Zanne AE (2009) Towards a worldwide wood economics spectrum. Ecol Lett 12:351–366
Cruiziat P, Cochard H, Améglio T (2002) Hydraulic architecture of trees: main concepts and results. Ann For Sci 59:723–752
Eller CB, Lima AL, Oliveira RS (2013) Foliar uptake of fog water and transport belowground alleviates drought effects in the cloud forest tree species, Drimys brasiliensis (Winteraceae). New Phytologist 199:151–162
Fauset S, Baker TR, Lewis SL, Feldpausch TR, Affum-Baffoe K, Foli EG, Hamer KC, Swaine MD (2012) Drought-induced shifts in the floristic and functional composition of tropical forests in Ghana. Ecol Lett 15:1120–1129
Gotsch S, Geiger E, Franco A, Goldstein G, Meinzer F, Hoffmann W (2010) Allocation to leaf area and sapwood area affects water relations of co-occurring savanna and forest trees. Oecologia 163:291–301
Gotsch SG, Asbjornsen H, Holwerda F, Goldsmith GR, Weintraub AE, Dawson TE (2014) Foggy days and dry nights determine crown-level water balance in a seasonal tropical montane cloud forest. Plant Cell Environ 37:261–272
Graham EA, Mulkey SS, Kitajima K, Phillips NG, Wright SJ (2005) Cloud cover limits net CO2 uptake and growth of a rainforest tree during tropical rainy seasons. Proc Natl Acad Sci 100:572–576
Grubb PJ (1977) Control of forest growth and distribution on wet tropical mountains: with Special Reference to Mineral Nutrition. Ann Rev Ecol Syst 8:83–107
Hao G-Y, Sack L, Wang A-Y, Cao K-F, Goldstein G (2010) Differentiation of leaf water flux and drought tolerance traits in hemiepiphytic and non-hemiepiphytic Ficus tree species. Funct Ecol 24:731–740
Kikuzawa K, Lechowicz MJ (2011) Ecology of leaf longevity. Springer, NewYork
Korner C (2007) The use of ‘altitude’ in ecological research. Trends Ecol Evol 22:569–574
Kunert N, Schwendenmann L, Hölscher D (2010) Seasonal dynamics of tree sap flux and water use in nine species in Panamanian forest plantations. Agric For Meteorol 150:411–419
Lamont B, Lamont H (2000) Utilizable water in leaves of 8 arid species as derived from pressure-volume curves and chlorophyll fluorescence. Physiol Plant 110:64–71
Leuschner C (2000) Are high elevations in tropical mountains arid environments for plants? Ecology 81:1425–1436
Martínez-Vilalta J, Sala A, Piñol J (2004) The hydraulic architecture of Pinaceae—a review. Plant Ecol 171:3–13
Martins SC (2010) Caracterização dos solos e serapilheira ao longo do gradiente altitudinal da Mata Atlântica, estado de São Paulo. entro de Energia Nuclear na Agricultura. USP, Piracicaba, p 156
McDowell N, Pockman WT, Allen CD, Breshears DD, Cobb N, Kolb T, Plaut J, Sperry J, West A, Williams DG, Yepez EA (2008) Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New Phytol 178:719–739
McDowell NG, Beerling DJ, Breshears DD, Fisher RA, Raffa KF, Stitt M (2011) The interdependence of mechanisms underlying climate-driven vegetation mortality. Trends Ecol Evol 26:523–532
Meinzer F (2003) Functional Convergence in plant responses to the environment. Oecologia 134:1–11
Meinzer FC, Andrade JL, Goldstein G, Holbrook NM, Cavelier J, Wright SJ (1999) Partitioning of soil water among canopy trees in a seasonally dry tropical forest. Oecologia 121:293–301
Meinzer FC, Goldstein G, Andrade JL (2001) Regulation of water flux through tropical forest canopy trees: do universal rules apply? Tree Physiol 21:19–26
Morellato PC, Talora DC, Takahasi A, Bencke CSC, Romera EC, Ziparro VB (2000) Phenology of Atlantic Rain Forest trees: a comparative study. Biotropica 32:811–823
Motzer T, Munz N, Küppers M, Schmitt D, Anhuf D (2005) Stomatal conductance, transpiration and sap flow of tropical montane rain forest trees in the southern Ecuadorian Andes. Tree Physiol 25:1283–1293
Myers N, Mittermeier RA, Mittermeier CG, Fonseca GAB, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403:853–858
Myneni RB, Yang W, Nemani RR, Huete AR, Dickinson RE, Knyazikhin Y, Didan K, Fu R, Juárez RIN, Saatchi SS, Hi Hashimoto, Ichii K, Shabanov NV, Tan B, Ratana P, Privette JL, Morisette JT, Vermote EF, Roy DP, Wolfe RE, Friedl MA, Running SW, Votava P, El-Saleous N, Devadiga S, Su Y, Salomonson VV (2007) Large seasonal swings in leaf area of Amazon rain forests. Proc Natl Acad Sci USA 104:4820–4823
Nadezhdina N (1999) Sap flow index as an indicator of plant water status. Tree Physiol 19:885–891
Niinemets Ü (2001) Global-scale climatic controls of leaf dry mass per area density, and thickness in trees and shrubs. Ecology 82:453–469
O’Brien JJ, Oberbauer SF, Clark DB (2004) Whole tree xylem sap flow responses to multiple environmental variables in a wet tropical forest. Plant Cell Environ 27:551–567
Oliveira R, Christoffersen B, de V. Barros F, Teodoro G, Bittencourt P, Brum-Jr M, Viani RG (2014a) Changing precipitation regimes and the water and carbon economies of trees. Theor Exp Plant Physiol 26:65–82
Oliveira RS, Eller CB, Bittencourt PRL, Mulligan M (2014b) The hydroclimatic and ecophysiological basis of cloud forest distributions under current and projected climates. Ann Bot 113:909–920
Oliveira-Filho AT, Fontes MA (2000) Patterns of floristic differentiation among Atlantic Forests in Southeastern Brazil and the influence of climate. Biotropica 32:793–810
Oren R, Zimmermann R, Terbough J (1996) Transpiration in upper amazonia floodplain and upland forests in response to drought-breaking rains. Ecology 77:968–973
Phillips OL, van der Heijden G, Lewis SL, López-González G, Aragão LEOC, Lloyd J, Malhi Y, Monteagudo A, Almeida S, Dávila EA, Amaral I, Andelman S, Andrade A, Arroyo L, Aymard G, Baker TR, Blanc L, Bonal D, de Oliveira ÁCA, Chao K-J, Cardozo ND, da Costa L, Feldpausch TR, Fisher JB, Fyllas NM, Freitas MA, Galbraith D, Gloor E, Higuchi N, Honorio E, Jiménez E, Keeling H, Killeen TJ, Lovett JC, Meir P, Mendoza C, Morel A, Vargas PN, Patiño S, Peh KS-H, Cruz AP, Prieto A, Quesada CA, Ramírez F, Ramírez H, Rudas A, Salamão R, Schwarz M, Silva J, Silveira M, Ferry Slik JW, Sonké B, Thomas AS, Stropp J, Taplin JRD, Vásquez R, Vilanova E (2010) Drought-mortality relationships for tropical forests. New Phytol 187:631–646
Pillar VD (1997) Multivariate exploratory analysis and randomization testing with MULTIV. Coenoses 12:145–148
Ponce-Reyes R, Reynoso-Rosales V-H, Watson J, VanDerWal J, Fuller RA, Pressey RL, Possingham HP (2012) Vulnerability of cloud forest reserves in Mexico to climate change. Nat Clim Change 2:448–452
Poorter L (2009) Leaf traits show different relationships with shade tolerance in moist versus dry tropical forests. New Phytol 181:890–900
Pounds JA, Fogden MPL, Campbell JH (1999) Biological response to climate change on a tropical mountain. Nature 398:611–615
Rada F, García-Núñez C, Ataroff M (2009) Leaf gas exchange in canopy species of a venezuelan cloud forest. Biotropica 41:659–664
Renninger HJ, Phillips N, Salvucci GD (2010) Wet- vs. dry-season transpiration in an amazonian rain forest palmiriartea deltoidea. Biotropica 42:470–478
Rosado BHP (2011) Ecologia funcional de árvores da Mata Atlântica: o papel de atributos morfológicos, grau de exposição da copa e altitude sobre o uso de água das espécies. Unicamp, Campinas, Thesis, p 163
Rosado BHP, de Mattos EA (2007) Variação temporal de características morfológicas de folhas em dez espécies do Parque Nacional da Restinga de Jurubatiba, Macaé, RJ, Brasil. Acta Botanica Brasilica 21:741–752
Rosado BHP, de Mattos EA (2010) Interspecific variation of functional traits in a CAM-tree dominated sandy coastal plain. J Veg Sci 21:43–54
Rosado BHP, Oliveira RS, Aidar MPM (2010) Is leaf water repellency related to vapor pressure and crown exposure in tropical forests? Acta Oecol 36:645–649
Rosado BHP, Martins AC, Colomeu TC, Oliveira RS, Joly CA, Aidar MPM (2011) Fine root biomass and root length density in a lowland and a montane tropical rain forest, SP, Brazil. Biota Neotropica 11:203–209
Rosado BHP, Oliveira RS, Joly CA, Aidar MPM, Burgess SSO (2012) Diversity in nighttime transpiration behavior of woody species of the Atlantic Rain Forest, Brazil. Agric For Meterol 158:13–20
Rosado BHP, Dias ATC, de Mattos EA (2013) Going back to basics: importance of ecophysiology when choosing functional traits for studying communities and ecosystems. Natureza Conservação 11:15–22
Ryser P (1996) The importance of tissue density for growth and life span of leaves and roots: a comparison of five ecologically contrasting grasses. Funct Ecol 10:717–723
Sack L, Frole K (2006) Leaf structural diversity is related to hydraulic capacity in tropical rain forest trees. Ecology 87:483–491
Sack L, Tyree M (2005) Leaf hydraulics and its implication in plant structure and function. In: Holbrook MN, Zwieniecki MA (eds) Vascular Transport in Plants. Elsevier Academic Press,NewYork pp 93–114
Santiago LS, Goldstein G, Meinzer FC, Fownes JH, Mueller-Dombois D (2000) Transpiration and forest structure in relation to soil waterlogging in a Hawaiian montane cloud forest. Tree Physiol 20:673–681
Sentelhas PC, Pereira AR, Marin FR, Angelocci LR, Alfonsi RR, Caramori PH, Swart S (1999) Balanços Hídricos Climatológicos do Brasil-500 balanços hídricos de localidades brasileiras. ESALQ, Piracicaba. Available at: http://www.bdclima.cnpm.embrapa.br/index.php
Silva-Dias MAE, Vidale PE, Blanco CMR (1995) Case study and numerical simulation of the summer regional circulation in São Paulo Brazil. Bound Layer Meteorol 74:371–388
Sousa Neto E, Carmo JB, Keller M, Martins SC, Alves LF, Vieira SA, Piccolo MC, Camargo P, Couto HTZ, Joly CA, Martinelli LA (2011) Soil-atmosphere exchange of nitrous oxide, methane and carbon dioxide in a gradient of elevation in the coastal Brazilian Atlantic forest. Biogeosciences 8:733–742
Still CJ, Foster PN, Schneider SH (1999) Simulating the effects of climate change on tropical montane cloud forests. Nature 398:608–610
Sutherland WJ, Freckleton RP, Godfray HCJ, Beissinger SR, Benton T, Cameron DD, Carmel Y, Coomes DA, Coulson T, Emmerson MC, Hails RS, Hays GC, Hodgson DJ, Hutchings MJ, Johnson D, Jones JPG, Keeling MJ, Kokko H, Kunin WE, Lambin X, Lewis OT, Malhi Y, Mieszkowska N, Milner-Gulland EJ, Norris K, Phillimore AB, Purves DW, Reid JM, Reuman DC, Thompson K, Travis JMJ, Turnbull LA, Wardle DA, Wiegand T (2013) Identification of 100 fundamental ecological questions. J Ecol 101:58–67
Tobin MF, Lopez OR, Kursar TA (1999) Responses of Tropical Understory Plants to a Severe Drought: tolerance and Avoidance of Water Stress1. Biotropica 31:570–578
Velázquez-Rosas N, Meave J, Vazquez-Santana S (2002) Elevational variation of leaf traits in montane rain forest tree species at La Chinantla, Southern Mexico. Biotropica 34:534–546
Vendramini F, Díaz S, Gurvich D, Wilson PJ, Thompson K, Hodgson JG (2002) Leaf traits as indicators of resource-use strategy in floras with succulent species. New Phytol 154:147–157
Westoby M, Falser DS, Moles AT, Wesk PA, Wright IJ (2002) Plant ecological strategies: some leading dimensions of variation between species. Ann Rev Ecol Syst 33:125–159
Witkowski ETF, Lamont BB (1991) Leaf specific mass confounds leaf density and thickness. Oecologia 88:486–493
Woodward FI (1993) The lowland-to-upland transition—modelling plant responses to environmental change. Ecol Appl 3:404–408
Wright IJ, Westoby M, Reich PB (2002) Convergence towards higher leaf mass per area in dry and nutrient-poor has different consequences for leaf life span. J Ecol 90:534–553
Wright IJ, Reich P, Westoby M, Ackerly D, Baruch Z, Bongers F, Cavender-bares J, Chapin T, Cornelissen JHC, Diemer M, Flexas J, Garnier E, Groom P, Gulias J, Hikosaka K, Lamont B, Lee T, Lusk C, Midgley J, Laure-Navas M, Niinemets Ü, Oleksyn J, Osada N, Poorter H, Poot P, Prior L, Pyankov V, Roumet C, Thomas SC, Tjoelker MG, Veneklaas EJ, Villar R (2004) The world wide leaf economics spectrum. Nature 428:821–827
Wright IJ, Falster DS, Pickup M, Westoby M (2006) Cross-species patterns in the coordination between leaf and stem traits and their implications for plant hydraulics. Physiol Plant 127:445–456
Acknowledgments
We are grateful to Kathy Steppe for organizing the 9th International Workshop on Sap Flow, which prompted the publication of this paper. We thank both reviewers for good critiques and suggestions which improved the paper. We thank E. McLean, P. Grierson and E. Veneklaas for good discussions, A.T.C. Dias and G.R. Winck for helping with some statistical analysis and H. Rocha and H. Freitas for providing rainfall data (FAPESP 08/58120-3). Special thanks go to A. Downey and A. Arias, from ICT International Pty Ltd, and R. Belinello for the great technical support. The authors were supported by grants from CNPq and the Biota-FAPESP Program - Projeto Temático Gradiente Funcional (03/12595-7). COTEC/IF 41.065/2005 and IBAMA/CGEN 093/2005.
Conflict of interest
SSOB has a commercial interest in the sap flow sensors used in this study but declares that this did not influence any decisions made in the course of this research. The other authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Braeuning.
An erratum to this article is available at http://dx.doi.org/10.1007/s00468-017-1527-5.
Rights and permissions
About this article
Cite this article
Rosado, B.H.P., Joly, C.A., Burgess, S.S.O. et al. Changes in plant functional traits and water use in Atlantic rainforest: evidence of conservative water use in spatio-temporal scales. Trees 30, 47–61 (2016). https://doi.org/10.1007/s00468-015-1165-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-015-1165-8