Abstract
Key message
We examined sexual differences in scaling relationships among twig components in dioecious species Populus cathayana Rehd, and explored the functional adaptation of twigs in female plants.
Abstract
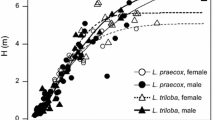
Since the evolution of reproductive requirements for disseminating pollen or producing seeds/fruits has led to sexual dimorphisms of twigs in dioecious species, different functional traits among twig components should exist between sexes. To explore sexual differences in scaling relationships among twig components in dioecious species, we took advantage of a field study of Populus cathayana Rehd, a dioecious tree native to China. Lamina mass and area, petiole mass and area, and stem mass were measured for the current-year terminal twigs in 62 (29 females and 33 males) mature P. cathayana trees along an altitudinal gradient (1,400–1,700 m) of the Xiaowutai Mountain, Hebei, north China. The scaling relationships within twig and leaf components were determined using the model type II regression method. Significantly positive correlations among lamina area and petiole mass or lamina mass were found in both male and female trees at the twig and leaf level, and scaling relationships between these traits differed between sexes. An allometric scaling relationship with a common slope <1.0 existed between total leaf area and stem mass, while isometric scaling relationships were between lamina area and petiole mass or lamina mass. Females had larger total leaf area per unit stem mass than males at the twig level, but a thinner and larger blade for a given petiole mass than males at the leaf level. Our results demonstrated that females tended to have more photosynthetic organ area per unit supporting tissue mass than males, which reflects functional adaptation of twigs in female plants to meet their specific reproductive needs.






Similar content being viewed by others
References
Ainsworth C (2000) Boys and girls come out to play: The molecular biology of dioecious plants. Ann Bot 86:211–221
Bond WJ, Honing M, Maze KE (1999) Seed size and seedling emergence: an allometric relationship and some ecological implications. Oecologia 120:132–136
Bressman EN (1932) Some dioecious plants. J Hered 23:67–69
Brouat C, Gibernau M, Amsellem L, McKey D (1998) Corner’s rules revisited: ontogenetic and interspecific patterns in leaf-stem allometry. New Phytol 139:459–470
Chen H, Niklas KJ, Yang DM, Sun SC (2009) The effect of twig architecture and seed number on seed size variation in subtropical woody species. New Phytol 183:1212–1221
Chen L, Han Y, Jiang H, Korpelainen H, Li C (2011) Nitrogen nutrient status induces sexual differences in responses to cadmium in Populus yunnanensis. J Exp Bot 62:5037–5050
Darwin C (1877) The different forms of flowers on plants of the same species. London, John Murray
Dawson TE, Ehleringer JR (1993) Gender-specific physiology, carbon isotope discrimination, and habitat distribution in boxelder, Acer negundo. Ecology 74:798–815
Dawson TE, Geber ME (1999) Sexual dimorphism in physiology and morphology. In: Geber MA, Dawson TE, Delph LF (eds) Gender and sexual dimorphism in flowering plants. Springer, New York, pp 175–215
Delph LF, Gehring JL, Arntz AM, Levri M, Frey FM (2005) Genetic correlations with floral display lead to sexual dimorphism in the cost of reproduction. Am Nat 166:S31–S41
Díaz S, Cabido M (2001) Vive la différence: plant functional diversity matters to ecosystem processes. Trends Ecol Evol 16:646–655
Donovan LA, Maherali H, Caruso CM, Huber H, de Kroon H (2011) The evolution of the worldwide leaf economics spectrum. Trends Ecol Evol 26:88–95
Eideh RA, Elkarmi A (2005) Allometric relationships of Malva parviflora growing in two different bioclimatic regions. J Plant Biol 48:319–325
Falster DS, Warton DI, Wright IJ (2006) SMATR: standardised major axis tests and routines. Version 2.0, Copyright 2006. http://www.bio.mq.edu.au/ecology/SMATR/index.html
Freeman DC, Klikoff LG, Harper KT (1976) Differential resource utilization by the sexes of dioecious plants. Science 193:597–599
Givnish TJ (1986) On the economy of plant form and function. Cambridge University Press, Cambridge
Givnish TJ (1987) Comparative studies of leaf form: assessing the relative roles of selective pressures and phylogenetic constraints. New Phytol 106:131–160
Gross KL (1984) Effects of seed size and growth form on seedling establishment of six monocarpic perennial plants. J Ecol 72:369–387
Gross KL, Soule JD (1981) Differences in biomass allocation to reproductive and vegetative structures of male and female plants of a dioecious, perennial herb, Silene alba (Miller) Krause. Am J Bot 68:801–807
Han Y, Wang L, Zhang X, Korpelainen H, Li C (2013) Sexual differences in photosynthetic activity, ultrastructure and phytoremediation potential of Populus cathayana exposed to lead and drought. Tree Physiol 33:1043–1060
Kavanagh PH, Lehnebach CA, Shea MJ, Burns KC (2011) Allometry of sexual size dimorphism in dioecious plants: do plants obey Rensch’s rule? Am Nat 178:596–601
Körner C, Bannister P, Mark AF (1986) Altitudinal variation in stomatal conductance, nitrogen content and leaf anatomy in different plant life forms in New Zealand. Oecologia 69:577–588
Li GY, Yang DM, Sun SC (2008) Allometric relationships between lamina area, lamina mass and petiole mass of 93 temperate woody species vary with leaf habit, leaf form and altitude. Funct Ecol 22:557–564
Liu ZL, Zheng CY, Fang JY (2004) Relationship between the vegetation type and topography in Mt. Xiaowutai, Hebei Province: a remote sensing analysis. Biodivers Sci 12:146–154 (in Chinese with English abstract)
Niinemets Ü, Kull O (1999) Biomass investment in leaf lamina versus lamina support in relation to growth irradiance and leaf size in temperate deciduous trees. Tree Physiol 19:349–358
Niinemets Ü, Portsmuth A, Tobias M (2006) Leaf size modifies support biomass distribution among stems, petioles and mid-ribs in temperate plants. New Phytol 171:91–104
Niinemets Ü, Portsmuth A, Tena D, Tobias M, Matesanz S, Valladares F (2007) Do we underestimate the importance of leaf size in plant economics? Disproportional scaling of support costs within the spectrum of leaf physiognomy. Ann Bot 100:283–303
Niklas K (1995) Size-dependent allometry of tree height, diameter and trunk-taper. Ann Bot 75:217–227
Niklas KJ (1999) A mechanical perspective on foliage leaf form and function. New Phytol 143:19–31
Niklas KJ, Enquist BJ (2002) Canonical rules for plant organ biomass partitioning and growth allocation. Am J Bot 89:812–819
Obeso JR (2002) The costs of reproduction in plants. New Phytol 155:321–348
Pickup M, Westoby M, Basden A (2005) Dry mass costs of deploying leaf area in relation to leaf size. Funct Ecol 19:88–97
Pitman ETG (1939) A note on normal correlation. Biometrika 31:9–12
Popp JW, Reinartz JA (1988) Sexual dimorphism in biomass allocation and clonal growth of Xanthoxylum americanum. Am J Bot 75:1732–1741
Queenborough SA, Burslem D, Garwood NC, Valencia R (2007) Determinants of biased sex ratios and inter-sex costs of reproduction in dioecious tropical forest trees. Am J Bot 94:67–78
Renner SS, Ricklefs RE (1995) Dioecy and its correlates in the flowering plants. Am J Bot 82:596–606
Shinozaki K, Yoda K, Hozumi K, Kira T (1964) A quantitative analysis of plant form—the pipe model theory. I. basic analysis. Jap J Ecol 14:97–105
Št’astná P, Klimešová J, Doležal J (2012) Altitudinal changes in the growth and allometry of Rumex alpinus. Alpine Bot 122:35–44
Sterck FJ, Gelder HAV, Poorter L (2006) Mechanical branch constraints contribute to life-history variation across tree species in a Bolivian forest. J Ecol 94:1192–1200
Sun SC, Jin DM, Shi PL (2006) The leaf size-twig size spectrum of temperate woody species along an altitudinal gradient: an invariant allometric scaling relationship. Ann Bot 97:97–107
Warton DI, Weber NC (2002) Common slope tests for bivariate errors-in-variables models. Biometrical J 44:161–174
Westoby M, Wright IJ (2003) The leaf size-twig size spectrum and its relationship to other important spectra of variation among species. Oecologia 135:621–628
Westoby M, Wright IJ (2006) Land-plant ecology on the basis of functional traits. Trends Ecol Evol 21:261–268
Westoby M, Falster DS, Moles AT, Vesk PA, Wright IJ (2002) Plant ecological strategies: some leading dimensions of variation between species. Annu Rev Ecol Syst 33:125–159
Wheelwright NT, Sinclair JP, Hochwender C, Janzen FJ (2012) Leaf size in three generations of a dioecious tropical tree, Ocotea tenera (Lauraceae): sexual dimorphism and changes with age. Am J Bot 99:1350–1355
White PS (1983a) Corner’s rules in eastern deciduous trees: allometry and its implications for the adaptive architecture of trees. Bull Torrey Bot Club 110:203–212
White PS (1983b) Evidence that temperate east North American evergreen woody plants follow Corner’s rules. New Phytol 95:139–145
Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, Cavender-Bares J, Chapin T, Cornelissen JHC, Diemer M, Flexas J, Garnier E, Groom PK, Gulias J, Hikosaka K, Lamont BB, Lee T, Lee W, Lusk C, Midgley JJ, Navas M, Niinemets Ü, Oleksyn J, Osada N, Poorter H, Poot P, Prior L, Pyankov VI, Roumet C, Thomas SC, Tjoelker MG, Veneklaas EJ, Villar R (2004) The worldwide leaf economics spectrum. Nature 428(6985):821–827
Wright IJ, Falster DS, Pickup M, Westoby M (2006) Cross-species patterns in the coordination between leaf and stem traits, and their implications for plant hydraulics. Physiol Plantarum 127:445–456
Xiang S, Wu N, Sun SC (2009) Within-twig biomass allocation in subtropical evergreen broad-leaved species along an altitudinal gradient: allometric scaling analysis. Trees-Struct Funct 23:637–647
Xu X, Peng GQ, Wu CC, Korpelainen H, Li CY (2008a) Drought inhibits photosynthetic capacity more in females than in males of Populus cathayana. Tree Physiol 28:1751–1759
Xu X, Yang F, Xiao XW, Zhang S, Korpelainen H, Li CY (2008b) Sex-specific responses of Populus cathayana to drought and elevated temperatures. Plant Cell and Environ 31:850–860
Xu X, Zhao HX, Zhang XL, Hänninen H, Korpelainen H, Li CY (2010) Different growth sensitivity to enhanced UV-B radiation between male and female Populus cathayana. Tree Physiol 30:1489–1498
Yan E, Wang X, Chang SX, He F (2013) Scaling relationships among twig size, leaf size and leafing intensity in a successional series of subtropical forests. Tree Physiol 33:609–617
Yang DM, Li GY, Sun SC (2008) The generality of leaf size versus number trade-off in temperate woody species. Ann Bot 102:623–629
Yang DM, Li GY, Sun SC (2009) The effects of leaf size, leaf habit, and leaf form on leaf/stem relationships in plant twigs of temperate woody species. J Veg Sci 20:359–366
Yang DM, Niklas KJ, Xiang S, Sun SC (2010) Size-dependent leaf area ratio in plant twigs: implication for leaf size optimization. Ann Bot 105:71–77
Yu PT, Liu HY, Cui HT (2002) Vegetation and its relation with climate conditions near the timberline of Beitai, the Xiaowutai Mts. Northern China. Chin J Appl Ecol 13:523–528 (in Chinese with English abstract)
Zhang S, Chen L, Duan B, Korpelainen H, Li C (2012) Populus cathayana males exhibit more efficient protective mechanisms than females under drought stress. Forest Ecol Manag 275:68–78
Author contribution statement
Yang, Y. performed the experiments and drafted the manuscript. He, X. revised the manuscript. Xu, X. designed the experiments and wrote the manuscript. Yang, D. conducted data analyses. All authors contributed to discussions of the results and implications at all stages.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (31170389) and the Innovative Team Foundation of Sichuan Provincial Department of Education (14TD0015).
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work. There is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of our manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. Guy.
Rights and permissions
About this article
Cite this article
Yang, Y., He, X., Xu, X. et al. Scaling relationships among twig components are affected by sex in the dioecious tree Populus cathayana . Trees 29, 737–746 (2015). https://doi.org/10.1007/s00468-014-1151-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-014-1151-6