Abstract
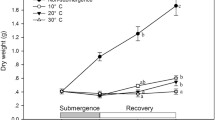
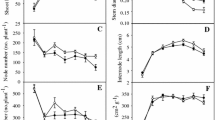
Melaleuca cajuputi is a woody plant of the Myrtaceae which is a dominant species in tropical peat swamps in southern Thailand, where the groundwater level fluctuates greatly. Although the current year seedlings are likely submerged, their adaptive responses have never been studied. The objective of the present study was to examine their responses to submergence, and especially their morphological and anatomical changes. Not only did the seedlings of M. cajuputi survive submergence for 56 days, but they could also increase their dry weight, shoot length, and leaf number during submergence. These growth responses to submergence indicate that the seedlings of M. cajuputi could make photosynthetic production under water. The leaves that developed under water were heterophyllous “aquatic leaves” that appear to represent adaptations to improve the uptake of gases from the water. Intercellular spaces in the stems and leaves were more strongly developed in the submerged seedlings than in non-submerged seedlings with the shoot and leaves in the air. The intercellular spaces appear to be schizogenous aerenchyma that facilitates gas exchange. The growth responses and anatomical responses in stems and leaves to submergence, which were found in M. cajuputi, are commonly known in herbaceous plants with amphibious characteristics, but had not been reported in woody plants. And our results suggest that M. cajuputi adapts to submergence similarly to other amphibious plants, thereby ensuring continuing biomass production.





Similar content being viewed by others
References
Bailey-Serres J, Voesenek L (2008) Flooding stress: acclimations and genetic diversity. Ann Rev Plant Biol 59:313–339
Blake ST (1968) A revision of Melaleuca leucadendron and its allies (Myrtaceae). Contrib Queens Herb 1:1–114
Bruni NC, Young JP, Dengler NG (1996) Leaf developmental plasticity of Ranunculus flabellaris in response to terrestrial and submerged environments. Can J Bot 74:823–837
Clevering OA, Van Vierssen W, Blom C (1995) Growth, photosynthesis and carbohydrate utilization in submerged Scirpus maritimus L. during spring growth. New Phytol 130:105–116
Colmer TD (2003) Long-distance transport of gases in plants: a perspective on internal aeration and radial oxygen loss from roots. Plant Cell Environ 26:17–36
Colmer TD, Pedersen O (2008) Oxygen dynamics in submerged rice (Oryza sativa). New Phytol 178:326–334
Colmer TD, Voesenek L (2009) Flooding tolerance: suites of plant traits in variable environments. Funct Plant Biol 36:665–681
Connell EL, Colmer TD, Walker DI (1999) Radial oxygen loss from intact roots of Halophila ovalis as a function of distance behind the root tip and shoot illumination. Aquat Bot 63:219–228
De Simone O, Haase K, Muller E, Junk WJ, Gonsior G, Schmidt W (2002) Impact of root morphology on metabolism and oxygen distribution in roots and rhizosphere from two Central Amazon floodplain tree species. Funct Plant Biol 29:1025–1035
Frost-Christensen H, Sand-Jensen K (1995) Comparative kinetics of photosynthesis in floating and submerged Potamogeton leaves. Aquat Bot 51:121–134
Frost-Christensen H, Jorgensen LB, Floto F (2003) Species specificity of resistance to oxygen diffusion in thin cuticular membranes from amphibious plants. Plant Cell Environ 26:561–569
He JB, Bogemann GM, van de Steeg HG, Rijnders J, Voesenek L, Blom C (1999) Survival tactics of Ranunculus species in river floodplains. Oecologia 118:1–8
Jung J, Lee SC, Choi HK (2008) Anatomical patterns of aerenchyma in aquatic and wetland plants. J Plant Biol 51:428–439
Kuwabara A, Ikegami K, Koshiba T, Nagata T (2003) Effects of ethylene and abscisic acid upon heterophylly in Ludwigia arcuata (Onagraceae). Planta 217:880–887
Lockhart CS (1996) Aquatic heterophylly as a survival strategy in Melaleuca quinquenervia (Myrtaceae). Can J Bot 74:243–246
Lynn DE, Waldren S (2001) Morphological variation in populations of Ranunculus repens from the temporary limestone lakes (Turloughs) in the West of Ireland. Ann Bot 87:9–17
Mommer L, Pedersen O, Visser EJW (2004) Acclimation of a terrestrial plant to submergence facilitates gas exchange under water. Plant Cell Environ 27:1281–1287
Mommer L, de Kroon H, Pierik R, Bogemann GM, Visser EJW (2005a) A functional comparison of acclimation to shade and submergence in two terrestrial plant species. New Phytol 167:197–206
Mommer L, Pons TL, Wolters-Arts M, Venema JH, Visser EJW (2005b) Submergence-induced morphological, anatomical, and biochemical responses in a terrestrial species affect gas diffusion resistance and photosynthetic performance. Plant Physiol 139:497–508
Mommer L, Wolters-Arts M, Andersen C, Visser EJW, Pedersen O (2007) Submergence-induced leaf acclimation in terrestrial species varying in flooding tolerance. New Phytol 176:337–345
Nabben RHM, Blom C, Voesenek L (1999) Resistance to complete submergence in Rumex species with different life histories: the influence of plant size and light. New Phytol 144:313–321
Nielsen SL, Sand-Jensen K (1993) Photosynthetic implications of heterophylly in Batrachium peltatum (Schrank) Presl. Aquat Bot 44:361–371
Osaki M, Watanabe T, Ishizawa T, Nilnond C, Nuyim T, Sittibush C, Tadano T (1998) Nutritional characteristics in leaves of native plants grown in acid sulfate, peat, sandy podzolic, and saline soils distributed in Peninsular Thailand. Plant Soil 201:175–182
Parolin P (2009) Submerged in darkness: adaptations to prolonged submergence by woody species of the Amazonian floodplains. Ann Bot 103:359–376
Pedersen O, Sand-Jensen K, Revsbech NP (1995) Diel pulses of O2 and CO2 in sandy lake-sediments inhabited by Lobelia dortmanna. Ecology 76:1536–1545
Pedersen O, Vos H, Colmer TD (2006) Oxygen dynamics during submergence in the halophytic stem succulent Halosarcia pergranulata. Plant Cell Environ 29:1388–1399
Rascio N (2002) The underwater life of secondarily aquatic plants: some problems and solutions. Crit Rev Plant Sci 21:401–427
Raskin I (1983) A method for measuring leaf volume, density, thickness, and internal gas volume. Hortscience 18:698–699
Salter J, Morris K, Bailey PCE, Boon PI (2007) Interactive effects of salinity and water depth on the growth of Melaleuca ericifolia Sm. (Swamp paperbark) seedlings. Aquat Bot 86:213–222
Sand-Jensen K, Frost-Christensen H (1999) Plant growth and photosynthesis in the transition zone between land and stream. Aquat Bot 63:23–35
Setter TL, Waters I, Wallace I, Bhekasut P, Greenway H (1989) Submergence of rice I. Growth and photosynthetic response to carbon dioxide enrichment of floodwater. Aust J Plant Physiol 16:251–264
Sorrell BK, Dromgoole FI (1987) Oxygen-transport in the submerged fresh-water macrophyte Egeria densa Planch. 1. Oxygen production, storage and release. Aquat Bot 28:63–80
Tahara K (2005) Aluminum tolerant mechanism in Melaleuca cajuputi. PhD thesis, Graduate School of Agricultural and life Sciences, University of Tokyo
Thomson CJ, Armstrong W, Waters I, Greenway H (1990) Aerenchyma formation and associated oxygen movement in seminal and nodal roots of wheat. Plant Cell Environ 13:395–403
Winkel A, Borum J (2009) Use of sediment CO2 by submersed rooted plants. Ann Bot 103:1015–1023
Wium-Andersen S, Andersen JM (1972) Carbon dioxide content of the interstitial water in the sediment of Grane Langsø, a Danish Lobelia lake. Limnol Oceanogr 17:943–947
Yamanoshita T (2001) Adaptation to flooding in a tropical peat swamp in Melaleuca cajuputi. PhD thesis, Graduate School of Agricultural and life Sciences, University of Tokyo
Yamanoshita T, Nuyim T, Masumori M, Tange T, Kojima K, Yagi H, Sasaki S (2001) Growth response of Melaleuca cajuputi to flooding in a tropical peat swamp. J For Res 6:217–219
Yamanoshita T, Masumori M, Yagi H, Kojima K (2005) Effects of flooding on downstream processes of glycolysis and fermentation in roots of Melaleuca cajuputi seedlings. J For Res 10:199–204
Acknowledgments
This study was supported in part by a Grant-in-Aid for Scientific Research (No. 19580164, No. 22580158) from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by T. Koike.
Rights and permissions
About this article
Cite this article
Tanaka, K., Masumori, M., Yamanoshita, T. et al. Morphological and anatomical changes of Melaleuca cajuputi under submergence. Trees 25, 695–704 (2011). https://doi.org/10.1007/s00468-011-0547-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-011-0547-9