Abstract
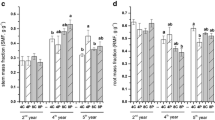
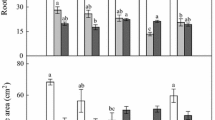
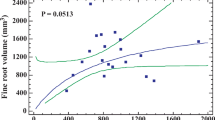
Thigmomorphogenesis is a well-studied process in agricultural crops and coniferous trees. Nevertheless, the effects on both shoot and root characteristics for deciduous woody species received little attention so far. In this study, the objective was to understand the effect of aboveground flexing treatments on the development of structural, mechanical and physiological root and shoot characteristics for two deciduous tree species, Black locust (Robinia pseudoacacia L.) and English oak (Quercus robur L.). Flexing treatments were performed using an electromechanical device with a rotating arm touching and bending the plants at regular intervals. A wide range of stem, shoot as well as root system characteristics was measured. The different flexing treatments altered above- and belowground plant development for both species, with strongest effects on Quercus and most significant differences between the control and the unidirectional flexing treatment. Some responses are in accordance with previous findings, such as stem eccentricity and reduced shoot elongation under unidirectional flexing, but others are renewing, such as the lower stomatal density and larger epidermal cell surface for the Quercus plants under variable flexing direction. Despite some common responses, both species frequently differed in the way they were affected. Belowground, Quercus plants under unidirectional flexing invested relatively more in their first order root and deeper second order roots, whereas Robinia plants allocated relatively more to fine root biomass and horizontal shallow roots. Both strategies potentially increased pull-out as well as overturning resistance in their own way. The presented findings are valid for young trees grown in small containers. Based on practical know-how and shortcomings experienced in the course of this experiment, methodological recommendations are formulated. We finally stress the complex variability in growth responses, especially for root systems, observed in different studies and related to dissimilarity in species, soil conditions, plant history or type of mechanical perturbation.





Similar content being viewed by others
References
Barthèlèmy D, Caraglio Y (2007) Plant architecture: a dynamic, multilevel and comprehensive approach to plant form, structure and ontogeny. Ann Bot 99:375–407
Berthier S, Stokes A (2005a) Phototrophic response induced by wind loading in Maritime pine seedlings (Pinus pinaster Aït.). J Exp Bot 56(413):851–856
Berthier S, Stokes A (2005b) Righting response of artificially inclined maritime pine (Pinus pinaster) seedlings to wind loading. Tree Physiol 26:73–79
Biddington NL (1986) The effects of mechanically-induced stress in plants: a review. Plant Growth Regul 4:103–123
Biddington NL, Dearman AS (1985) The effect of mechanically induced stress on the growth of cauliflower, lettuce and celery seedlings. Ann Bot 55:109–119
Biro RL, Hunt ERJ, Erner Y, Jaffe MF (1980) Thigmomorphogenesis: changes in cell division and elongation in the internodes of mechanically-perturbed or ethrel-treated bean plants. Ann Bot 45:655–664
Bischetti GB, Chiaradia EA, Simonato T, Speziali B, Vitali B, Vullo P, Zocco A (2005) Root strength and root area ratio of forest species in Lombardy (Northern Italy). Plant Soil 278:11–22
Buchanan BB, Gruissem W, Jones R (2000) Biochemistry & molecular biology of plants. The American Society of Plant Physiologists, Rockville
Chiatante D, Di Iorio A, Scippa GS (2007) Modification of root architecture in woody plants is possible for the presence of two different mechanisms of lateral root production: the effect of slope in Spartium junceum L. seedlings. Plant Bios 141:502–511
Cofie P, Koolen AJ (2001) Test speed and other factors affecting the measurements of tree root properties used in soil reinforcement models. Soil Tillage Res 63:51–56
Coppin NJ, Richards IG (1990) Use of vegetation in civil engineering. Butterworths, London
Coutand C, Julien JL, Moulia B, Mauget JC, Guitard D (2000) Biomechanical study of the effect of a controlled bending on tomato stem elongation: global mechanical analysis. J Exp Bot 51:1813–1824
Danjon F, Reubens B (2008) Assessing and analyzing 3D architecture of woody root systems, a review of methods and applications in tree and soil stability, resource acquisition and allocation. Plant Soil 303:1–34
Danjon F, Bert D, Godin C, Trichet P (1999a) Structural root architecture of 5-year-old Pinus pinaster measured by 3D digitising and analysed with Amapmod. Plant Soil 217:49–63
Danjon F, Sinoquet H, Godin C, Colin F, Drexhage M (1999b) Characterisation of structural tree root architecture using 3D digitising and Amapmod software. Plant Soil 211:241–258
Danjon F, Fourcaud T, Bert D (2005) Root architecture and wind-firmness of mature Pinus pinaster. New Phytol 168:387–400
De Baets S, Poesen J, Reubens B, Wemans K, De Baerdemaeker J, Muys B (2008) Root tensile strength and root distribution of typical Mediterranean plant species and their contribution to soil shear strength. Plant Soil 305:207–226
Dunn GH, Dabney SM (1996) Modulus of elasticity and moment of inertia of grass hedge stems. Trans Asae 39:947–952
Dupuy L, Fourcaud T, Lac P, Stokes A (2003) Modelling the influence of morphological and mechanical properties on the anchorage of root systems. In: Proceedings of the international conference on wind effects on trees. University of Karlsruhe, Germany
Dupuy L, Fourcaud T, Stokes A (2005) A numerical investigation into the influence of soil type and root architecture on tree anchorage. Plant Soil 278:119–134
Dupuy L, Fourcaud F, Lac P, Stokes A (2007) A generic 3D finite element model of tree anchorage integrating soil mechanics and real root system architecture. Am J Bot 94:1506–1514
Ennos AR (1993) The scaling of root anchorage. J Theo Biol 161:61–75
Ennos AR, Crook MJ, Grimshaw C (1993) A comparative study of the anchorage systems of Himalyan Balsam Impatiens glandulifera and mature sunflower Helicanthus annuus. J Exp Bot 258:133–146
Fluch S, Olmo CC, Tauber S, Stierschneider M, Kopecky D, Reichenauer TG, Matusikova I (2008) Transcriptomic changes in wind-exposed poplar leaves are dependent on developmental stage. Planta 228:757–764
Fourcaud T, Li JN, Zhang Z, Stokes A (2008) Understanding the impact of root morphology on overturning mechanisms: a modelling approach. Ann Bot 101:1267–1280
Frost CJ, Hunter MD (2008) Herbivore-induced shifts in carbon and nitrogen allocation in red oak seedlings. New Phytol 178(4):835–845
Genet M, Stokes A, Salin F, Mickovski S, Fourcaud T, Dumail JF, Van Beek R (2005) The influence of cellulose content on tensile strength in tree roots. Plant Soil 278:1–9
Gomez KA, Gomez AA (1984) Statistical procedures for agricultural research. Wiley, New York
Goodman AM, Ennos AR (1998) Responses of the root systems of sunflower and maize to unidirectional stem flexure. Ann Bot 82:347–357
Goodman AM, Ennos AR (1999) The effects of soil bulk density on the morphology and anchorage mechanics of the root systems of sunflower and maize. Ann Bot 83:293–302
Gray DH, Sotir RB (1996) Biotechnical and soil bioengineering slope stabilization: a practical guide for erosion control. Wiley, Chichester
Grime JP (1979) Plant strategies and vegetation processes. Wiley, Chichester
Grime JP, Hunt R (1975) Relative growth-rate—its range and adaptive significance in a local flora. J Ecol 63:393–422
Hallé F (1978) Architectural variation at specific level of tropical trees. In: Tomlinson PB, Zimmermann MH (eds) Tropical trees as living systems. Cambridge University Press, Cambridge, pp 209–211
Harrington CA, DeBell DS (1996) Above- and below-ground characteristics associated with wind toppling in a young Populus plantation. Trees 11:109–118
Holbrook N, Putz F (1989) Influence of neighbours on tree form: effects of lateral shade and prevention of sway on the allometry of Liquidambar styraciflua (sweet gum). Am J Bot 76:1740–1749
Hunt ERJ, Jaffe MJ (1980) Thigmomorphogenesis: the interaction of wind and temperature in the field on the growth of Phaseolus vulgaris L. Ann Bot 45:665–672
Jaffe MJ (1973) Thigmomorphogenesis—response of plant-growth and development to mechanical stimulation—with special reference to Bryonia dioica. Planta 114:143–157
Khuder H (2007) Etude de l’effet d’une pente sur l’architecture et les propriétés mécaniques des systèmes racinaires de semis d’arbres. PhD thesis, Université de Bordeaux, France
Khuder H, Danjon F, Stokes S, Fourcaud T (2006) Growth response and root architecture of Black locust seedlings growing on slopes and subjected to mechanical perturbation. In: Salmen L (ed) Proceedings of the 5th plant biomechanics conference. Stockholm, Sweden, pp 299–303
Khuder H, Stokes A, Danjon F, Gouskou K, Lagane F (2007) Is it possible to manipulate root anchorage in young trees? Plant Soil 294:87–102; erratum 295:293–295
Larsena DR, Johnson PS (1998) Linking the ecology of natural oak regeneration to silviculture. For Ecol Manag 106(1):1–7
Lindstrom A, Rune G (1999) Root deformation in plantations of container-grown Scots Pine trees: effects on root growth, tree stability and stem straightness. Plant Soil 217:29–37
Lundqvist L, Valinger E (1996) Stem diameter growth of Scots Pine trees after increased mechanical load in the crown during dormancy and (or) growth. Ann Bot 77:59–62
Mattheck C, Teschner M, Schafer J (1997) Mechanical control of root growth: a computer simulation. J Theo Biol 184:261–269
Mattia C, Bischetti GB, Gentile F (2005) Biotechnical characteristics of root systems of typical Mediterranean species. Plant Soil 278:23–32
Mickovski SB, Ennos AR (2003) The effect of unidirectional stem flexing on shoot and root morphology and architecture in young Pinus sylvestris trees. Can J For Res 33:2202–2209
Neill RL, Neill DM, Frye BF (1990) Is there a correlation between rainfall amounts and the number of stomata in Cottonwood leaves. Am Biol Teacher 52:48–49
Nicoll BC, Ray D (1996) Adaptive growth of tree root systems in response to wind action and site conditions. Tree Physiol 16:891–898
Nicoll BC, Gardiner BA, Peace AJ (2008) Improvements in anchorage provided by the acclimation of forest trees to wind stress. Forestry 81:389–398
Niklas KJ (1999) Variations of the mechanical properties of Acer saccharum roots. J Exp Bot 50:193–200
Niklas KJ, Spatz HC (2000) Wind-induced stresses in Cherry trees: evidence against the hypothesis of constant stress levels. Trees 14:230–237
Nilaweera NS, Nutalaya P (1999) Role of tree roots in slope stabilisation. Bull Eng Geol Environ 57:337–342
Norris JE (2005) Root reinforcement by Hawthorn and Quercus roots on a highway cut-slope in Southern England. Plant Soil 278:43–53
Oppelt AL, Kurth W, Godbold DL (2001) Topology, scaling relations and Leonardo’s rule in root systems from African tree species. Tree Physiol 21:117–128
Pruyn ML, Ewers BJ, Telewski FW (2000) Thigmomorphogenesis: changes in the morphology and mechanical properties of two Populus hybrids in response to mechanical perturbation. Tree Physiol 20:535–540
Reubens B, Poesen J, Danjon F, Geudens G, Muys B (2007) The role of fine and coarse roots in shallow slope stability and soil erosion control with a focus on root system architecture: a review. Trees 21:385–402
Smit AL, Bengough AG, Engels C, van Noordwijk M, Pellerin S, van de Geijn SC (2000) Root methods: a handbook. Springer, Berlin
Smith VC, Ennos AR (2003) The effects of air flow and stem flexure on the mechanical and hydraulic properties of the stems of sunflowers Helianthus annuus L. J Exp Bot 54:845–849
Stokes A, Fitter AH, Coutts MP (1995) Responses of young trees to wind and shading: effects on root architecture. J Exp Bot 46:1139–1146
Stokes A, Ball J, Fitter AH, Brain P, Coutts MP (1996) An experimental investigation of the resistance of model root systems to uprooting. Ann Bot 78:415–421
Stokes A, Nicoll BC, Coutts MP, Fitter AH (1997) Responses of young Sitka Spruce clones to mechanical perturbation and nutrition: effects on biomass allocation, root development, and resistance to bending. Can J For Res 27:1049–1057
Tamasi E, Stokes A, Lasserre B, Danjon F, Berthier S, Fourcaud T, Chiatante D (2005) Influence of wind loading on root system development and architecture in Quercus (Quercus robur L.) seedlings. Trees 19:374–384
Telewski FW (1990) Growth, wood density, and ethylene production in response to mechanical perturbation in Pinus taeda. Can J For Res 20:1277–1282
Telewski FW (1995) Wind-induced physiological and developmental responses in trees. In: Coutts M, Grace J (eds) Wind and trees. Cambridge University Press, Cambridge, pp 237–263
Telewski FW, Jaffe MJ (1981) Thigmomorphogenesis: changes in the morphology and chemical composition induced by mechanical perturbation in 6-month-old Pinus taeda seedlings. National research council of Canada, Canada, pp 380–387
Telewski FW, Jaffe MJ (1986a) Thigmomorphogenesis: anatomical, morphological and mechanical analysis of genetically different sibs of Pinus taeda in response to mechanical perturbation. Phys Plant 66:219–226
Telewski FW, Jaffe MJ (1986b) Thigmomorphogenesis: field and laboratory studies of Abies fraseri in response to wind or mechanical perturbation. Phys Plant 66:211–218
Telewski FW, Pruyn ML (1998) Thigmomorphogenesis: a dose response to flexing in Ulmus americana seedlings. Tree Physiol 18:65–68
Vincent JFV (1992) Biomechanics-materials: a practical approach. Oxford University Press, Oxford
Acknowledgments
This work was supported by the Flemish Interuniversity Council (VLIR) and the K. U. Leuven Research Fund. We thank Jean-Pierre Van Cuyck, Roland Reekmans, Dirk Bastiaensen, Kristof Mertens and Flip Bamelis for the technical and other supports in the experimental setup and the design of a suitable method for the mechanical tests. Special thanks also go to Lionel Dupuy for his advice related to the ArchiRoot software, and to the Forecoman colleagues, the editor and the anonymous reviewer for their constructive comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Zwieniecki.
Rights and permissions
About this article
Cite this article
Reubens, B., Pannemans, B., Danjon, F. et al. The effect of mechanical stimulation on root and shoot development of young containerised Quercus robur and Robinia pseudoacacia trees. Trees 23, 1213–1228 (2009). https://doi.org/10.1007/s00468-009-0360-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-009-0360-x