Abstract
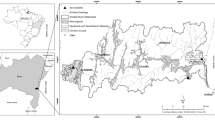
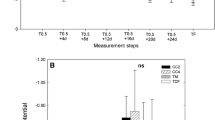
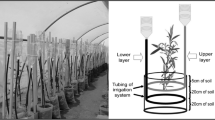
The purpose of this work is to increase ecological understanding of Avicennia germinans L. and Laguncularia racemosa (L.) Gaertn. F. growing in hypersaline habitats with a seasonal climate. The area has a dry season (DS) with low temperature and vapour pressure deficit (vpd), and a wet season (WS) with high temperature and slightly higher vpd. Seasonal patterns in interstitial soil water salinity suggested a lack of tidal flushing in this area to remove salt along the soil profile. The soil solution sodium/potassium (Na+/K+) ratio differed slightly along the soil profile during the DS, but during the WS it was significantly higher at the soil surface. Diurnal changes in xylem osmolality between predawn (higher) and midday (lower) were observed in both species. However, A. germinans had higher xylem osmolality compared to L. racemosa. Xylem Na+/K+ suggested higher selectivity of K+ over Na+ in both species and seasons. The water relations parameters derived from pressure–volume P–V curves were relatively stable between seasons for each species. The range of water potentials (Ψ), measured in the field, was within estimated values for turgor maintenance from P–V curves. Thus the leaves of both species were osmotically adapted to maintain continued water uptake in this hypersaline mangrove environment.





Similar content being viewed by others
References
Amtman A, Sanders D (1999) Mechanisms of Na+ uptake by plant cells. Adv Bot Res 29:75–112
Ball MC (1988) Salinity tolerance in the mangrove Aegiceras corniculatum and Avicennia marina. I. Water use in relation to growth, carbon partitioning, and salt balance. Aust J Plant Physiol 15:447–464
Ball MC (1996) Comparative ecophysiology of mangrove forest and tropical lowland moist rainforest. In: Mulkey S, Chazdon RL, Smith AP (eds) Tropical forest plant ecophysiology. Chapman and Hall, New York, pp 461–496
Ball MC, Passioura JB (1994) Carbon gain in relation to water use: photosynthesis in mangroves. In: Schulze E-D, Caldwell MM (eds) Ecophysiology of photosynthesis. Springer, Berlin, Heidelberg New York, pp 247–259
Ball MC, Pidsley SM (1995) Growth responses to salinity in relation to distribution in two mangrove species, Sonneratia alba and S. lanceolata, in northern Australia. Funct Ecol 9:77–85
Ball MC, Sobrado MA (1999) Ecophysiology of mangroves: challenges in linking physiological process with patterns in forest structure: In: Press MC, Scholes JD, Baker MG (eds) Advances in plant physiological ecology. Blackwell Science, Oxford, pp 331–346
Ball MC, Cowan IR, Farquhar GD (1988) Maintenance of leaf temperature and the optimization of carbon gain in relation to water loss in a tropical mangrove forest. Aust J Plant Physiol 15:263-276
Boyer JB (1967) Matric potential of leaves. Plant Physiol 42:213–217
Boyer JS (1969) Measurements of the water status of plants. Annu Rev Plant Physiol 20:351–364
Cintrón G, Lugo AE, Pool DJ, Morris G (1978) Mangroves of arid environments in Puerto Rico and adjacent islands. Biotropica 10:110–121
Cram JW, Torr PG, Rose DA (2002) Salt allocation during leaf development and leaf fall in mangroves. Trees 16:112–119
Ehleringer JR, Phillips SL, Comstock JP (1992) Seasonal variation in the carbon isotopic composition of desert plants. Funct Ecol 6:396–404
Feller IC, Whigham DF, McKee KL, O'Neill JP (2002) Nitrogen vs. phosphorous limitation across an ecotonal gradient in mangrove forest. Biogeochemistry 62:145–175
Feller IC, Whigham DF, McKee KL, Lovelock CE (2003) Nitrogen limitation of growth and nutrient dynamics in a disturbed mangrove forest, Indian River Lagoon, Florida. Oecologia 134:405–414
Field C (1998) Rationales and practices of mangrove afforestation. Mar Freshwater Res 49:353–358
Jones MM, Turner NS, Osmond CB (1981) Mechanisms of drought resistance. In: Paleg LG, Aspinal D (eds) The physiology and biochemistry of drought resistance in plants. Academic Press, Sydney, pp 15–37
Kathiresan K, Bingham BL (2001) Biology of mangroves and mangrove ecosystems. Adv Mar Biol 40:81–251
Kramer PJ, Boyer JS (1995) Water relations of plants and soils. Academic Press, San Diego
Kubiske ME, Abrams MD (1991) Seasonal, diurnal and rehydration-induced variation of pressure–volume relationship in Pseudotsuga meinziesii. Physiol Plant 83:107–116
Ladiges PY (1975) Some aspects of tissue water relations in three populations of Eucalyptus viminalis Labill. New Phytol 75:53–62
Lichtenthaler HK, Wellburn AR (1983) Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem Soc Trans 11:591–592
Lin G, Sternberg L (1992a) Differences in morphology, carbon isotope ratios, and photosynthesis between scrub and fringe mangroves in Florida, USA. Aquat Bot 42:303–313
Lin G, Sternberg L (1992b) Effects of growth form, salinity, nutrient and sulphide on photosynthesis, carbon isotope discrimination and growth of red mangrove (Rhizophora mangle L.) Aust J Plant Physiol 19:509–517
Lovelock CE, Feller IC (2003) Photosynthetic performance and resource utilization of two mangrove species coexisting in a hypersaline scrub forest. Oecologia 134:455–462
López-Portillo J, Ezcurra E (1989) Zonation in mangrove and salt marsh vegetation at Laguna de Mecoacán, México. Biotropica 21:107–114
Lugo AE, Snedaker SC (1974) The ecology of mangroves. Annu Rev Ecol Syst 5:39–64
McKee KL (1995) Interspecific variation in growth, biomass partitioning, and defensive characteristics of neotropical mangrove seedlings, responses to light and nutrient availability. Am J Bot 82:299–307
McKee KL, Meldelssohn IA, Hester MW (1988) Reexamination of pore water sulphide concentration and redox potentials near the aerial roots of Rhizophora mangle and Avicennia germinans. Am J Bot 75:1352–1359
Medina E (1999) Mangrove physiology: the challenge of salt, heat and light stress under recurrent flooding. In: Yánez-Arancibia A, Lara-Domínguez AL (eds) Ecosystemas de manglar en América tropical. Instituto de Ecología A.C. Xalapa, México. UICN/ORMA Costa Rica NOAA/NMFS, Silver Spring, MD, pp 109–126
Medina E, Francisco M (1997) Osmolality and δ13C of leaf tissue of mangrove species from environments of contrasting rainfall and salinity. Estuarine Coast Shelf Sci 45:337–344
Miller RO (1998) Microwave digestion of plant tissue in a closed vessel. In: Kalra YP (ed) Handbook and reference methods for plant analysis. CRC Press, New York, pp 69–74
Monteith JL, Unsworth MH (1990) Principles of environmental physics. Edward Arnold, London
Munns R (1985) Na+, K+ and Cl− in xylem sap flowing to shoots of NaCl-treated barley. J Exp Bot 36:1032–1042
Munns R (1988) Effect of high external NaCl concentrations on ion transport within shoot of Lupinus alba. I. Ions on xylem sap. Plant Cell Environ 11:283–289
Passioura JB (1980) The meaning of matric potential. J Exp Bot 31:1161–1169
Passioura JB, Ball MC, Knight JH (1992) Mangroves may salinize the soil and so doing limit their transpiration rate. Funct Ecol 6:476–481
Pate JS (1976) Nutrients and metabolites fluids recovered from xylem and phloem: significance in relation to long-distance transport in plants. In: Warlaw IF, Passioura JB (eds) Transport and transfer process in plants. Academic Press, New York, pp 253–281
Pitman MG (1965) Sodium and potassium uptake by seedlings of Hordeum vulgaris. Aust J Biol Sci 18:10–24
Popp M (1984) Chemical composition of Australian mangroves. I. Low molecular weight carbohydrates. Z Pflanzenphysiol 113:411–421
Popp M, Larher F, Weigel P (1984) Chemical composition of Australian mangroves. III. Free amino acids, total methylated anion compounds and total nitrogen. Z Pflanzenphysiol 114:15–25
Rada F, Goldstein G, Orozco A Montilla M, Zabala O, Azocar A (1989) Osmotic and turgor relations of three mangrove species. Aust J Plant Physiol 16:477–486
Richter H (1997) Water relations of plants in the field: some comments on the measurement of selected parameters. J Exp Bot 48:1–7
Saenger P, Specht MM, Spetcht RL, Chapman VJ (1977) Mangal and coastal salt-marsch communities in Australasia. In: Chapman VJ (ed) Wet coastal ecosystem. Elsevier, Amsterdam, pp 293–345
Scholander PF (1968) How mangroves desalinate seawater. Physiol Plant 21:251–261
Scholander PF, Hammel HT, Hemmingsen EA, Garey W (1962) Salt balance in mangroves. Plant Physiol 37:722–729
Scholander PF, Bradstreet ED, Hammel HT, Hemmingsen EA (1966) Sap concentration in halophytes and some other species. Plant Physiol 41:529–532
Šesták, Z (1985) Chlorophylls and carotenoids during leaf ontogeny. In: Šesták Z (ed) Photosynthesis during leaf development. Academia Praha/Dr W. Junk Publ., Dordrecht, pp 76–106
Sobrado MA (1999) Drought effect on photosynthesis of the mangrove Avicennia germinans under contrasting salinities. Trees 13:125–130
Sobrado MA (2000) Relation of water transport to leaf gas exchange properties in three mangrove species. Trees 14:258–262
Sobrado MA (2001a) Hydraulic properties of a mangrove Avicennia germinans as affected by NaCl. Biol Plantarum 44:435–438
Sobrado MA (2001b) Effect of high external NaCl concentration on the osmolality of xylem sap, leaf tissue and leaf glands secretion of the mangrove Avicennia germinans (L.). Flora 196:63–70
Sobrado MA (2002) Effect of drought on leaf gland secretion of the mangrove Avicennia germinans L. Trees 16:1–4
Sobrado MA (2004) Influence of external salinity on the osmolality of xylem sap, leaf tissue and leaf gland secretion of the mangrove Laguncularia racemosa (L.) Gaertn. Trees 18:422–427
Sokal RR, Rohlf FJ (1969) Biometry. Freeman, San Francisco
Suárez N, Sobrado MA, Medina E (1998) Salinity effects on the leaf water relations components and ion accumulation patterns in Avicennia germinans L. seedlings. Oecologia 114:299–304
Tomlinson PB (1986) The botany of mangroves. Cambridge University Press, London
Turner NC (1981) Techniques and experimental approaches for the measurements of plant water status. Plant Soil 58:339–366
Tyree MT, Hammel M (1982) The measurements of the turgor pressure and water relations of plants by the pressure-bomb technique. J Exp Bot 23:267–283
Waisel Y, Eshel A, Agami M (1986) Salt balance of leaves of the mangrove Avicennia marina. Physiol Plant 67:67–72
Acknowledgements
This is a Smithsonian Marine Station (SMS) Contribution No. 663, Southeast Environmental Research Center Contribution No. 330 and Universidad Simón Bolívar (DID-FT-2005). We are pleased to thank Dr. V. Paul for giving us the opportunity to conduct this study at SMS, J. Kaminski, H. Reichardt, J. Piraino and W. Lee for logistic and technical support at the SMS at Fort Pierce, as well as the referees and Professor E. Medina (IVIC, Venezuela) for helpful suggestions on this manuscript. Ewe was in part supported by the National Science Foundation (DEB-9910514) during this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. Millard
Rights and permissions
About this article
Cite this article
Sobrado, M.A., Ewe, S.M.L. Ecophysiological characteristics of Avicennia germinans and Laguncularia racemosa coexisting in a scrub mangrove forest at the Indian River Lagoon, Florida. Trees 20, 679–687 (2006). https://doi.org/10.1007/s00468-006-0083-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-006-0083-1