Abstract
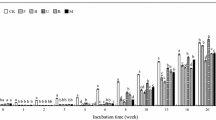
Seedlings of yellow birch (Betula alleghaniensis Britton) and sugar maple (Acer saccharum Marsh.) were grown for 2 years in mono-culture and mixed-culture and at three fertility levels. Following the second growing season, senescent leaves were analysed for N concentration, acid hydrolysable substances (AHS), and nonhydrolysable remains (NHR). A litter sub-sample was then inoculated with indigenous soil microflora, incubated 14 weeks, and mass loss was measured. Litter-N was significantly higher at medium than at poor fertility, as well as in yellow birch than in sugar maple litter. The species effect on litter-N increased with increasing fertility. At medium fertility, litter-N of sugar maple litter was lower in mixed-culture than in mono-culture. AHS, NHR as well the NHR/N ratio were significantly higher in yellow birch than in sugar maple litter. At medium fertility, the NHR/N ratio of sugar maple litter was significantly lower in mono-culture than in mixed-culture. Mass loss was significantly greater at medium and rich fertility than at poor fertility, and in yellow birch than in sugar maple litter. At poor fertility, mixed-litter decomposed at a rate comparable to yellow birch, whereas at medium and rich fertility, mixed-litter decomposed at a rate comparable to sugar maple. There was a significant positive relationship between litter-N and mass loss. A similar positive relationship between NHR and mass loss was presumed to be a “species” effect on decomposition. Results support the hypothesis that species × fertility and species × mixture interactions can be important determinants of litter quality and, by implication, of site nutrient cycling.



Similar content being viewed by others
References
Austin AT, Vitousek PM (2000) Precipitation, decomposition and litter decomposability of Metrosideros polymorpha in native forests on Hawaii. J Ecol 88:129–138
Beaudet M, Messier C, Hilbert DW, Lo E, Wang ZM, Lechowicz MJ (2000) Leaf- and plant-level carbon gain in yellow birch, sugar maple, and beech seedlings from contrasting forest light environments. Can J For Res 30:390–404
Berg B, Hannus K, Popoff T, Theander O (1982) Changes in organic chemical components of needle litter during decomposition. I. Long-term decomposition in Scots pine forest. Can J Bot 60:1310–1319
Bradley RL (2002) Dynamics of nitrogen associated to acid insoluble substances derived from plant litter. Commun Soil Sci Plant Anal 33:1277–1290
Bradley RL, Fyles JW (1996) Interactions between tree seedling roots and humus forms in the control of soil C and N cycling. Biol Fertil Soils 23:70–77
Bradley RL, Titus BD, Fyles JW (1997) Nitrogen acquisition and competitive ability of Kalmia angustifolia L., Paper Birch (Betula Papyrifera Marsh.) and Black Spruce (Picea Mariana (Mill.) Bsp) seedlings grown on different humus forms. Plant Soil 195:209–220
Carreiro MM, Sinsabaugh RL, Repert DA, Parkhurst DF (2000) Microbial enzyme shifts explain litter decay responses to simulated nitrogen deposition. Ecology 81:2359–2365
Coleman DC, Crossley DA (1996) Fundamentals of soil ecology. Academic, San Diego
Ellsworth DS (1998) Nitrogen additions affects leaf nutrition and photosynthesis in sugar maple in a nutrient-poor northern Vermont forest. In: Horsley SB, Long RP (eds) Proceedings of the international symposium on sugar maple ecology and health, pp 98–105
Finzi AC, Canham CD (2000) Sapling growth in response to light and nitrogen availability in a southern New England forest. For Ecol Manage 131:153–165
Fyles JW, Fyles IH (1993) Interaction of Douglas-fir with red alder and salal foliage litter during decomposition. Can J For Res 23:358–361
Kirk TK, Fenn P (1982) Formation and action of the ligninolytic system in basidiomycetes. Br Mycol Soc 4:67–89
Magill AH, Aber JD (1998) Long-term effects of experimental nitrogen additions on foliar litter decay and humus formation in forest ecosystems. Plant Soil 203:301–311
Malcolm DC (1987) Nitrogen supply for spruce on infertile sites (an ecological problem). The Leslie L. Schaffer lectureship in forest science, Vancouver, BC, Canada
McClaugherty CA, Pastor J, Aber JD, Melillo JM (1985) Forest litter decomposition in relation to soil nitrogen dynamics and litter quality. Ecology 66:266–275
Melillo JM, Aber JD, Muratore JF (1982) Nitrogen and lignin control of hardwood leaf litter decomposition dynamics. Ecology 63:621–626
Melillo JM, Aber JD, Linkins AE, Ricca A, Fry B, Nadelhoffer KJ (1989) Carbon and nitrogen dynamics along the decay continuum: plant litter to soil organic matter. Plant Soil 115:189–198
Miles J, Young WF (1980) The effects on heathland and moorland soils in Scotland and Northern England following colonization by birch (Betula spp.). Bull Ecol 11:233–242
Moore TR, Trofymow JA, Taylor B, Prescott C, Camire C, Duschene L, Fyles J, Kozak L, Kranabetter M, Morrison I, Siltanen M, Smith S, Titus B, Visser S, Wein R, Zoltai S (1999) Litter decomposition rates in Canadian forests. Global Change Biol 5:75–82
Preston CM, Trofymow JA, Sayer BG, Niu JN (1997) C-13 nuclear magnetic resonance spectroscopy with cross-polarization and magic-angle spinning investigation of the proximate-analysis fractions used to assess litter quality in decomposition studies. Can J Bot 75:1601–1613
Rothe A, Binkley D (2001) Nutritional interactions in mixed species forests: a synthesis. Can J For Res 31:1855–1870
Ryan MG, Melillo JM, Ricca A (1990) A comparison of methods for determining proximate carbon fractions of forest litter. Can J For Res 20:166–171
SAS (1998) SAS/Stat User’s Guide. Release 6.12. SAS Institute, Cary
Scowcroft PG, Turner DR, Vitousek PM (2000) Decomposition of Metrosideros polymorpha leaf litter along elevational gradients in Hawaii. Global Change Biol 6:73–85
Soil Classification Working Group (1998) The Canadian system of soil classification, 3rd edn. Agriculture and Agri-food Canada, Research Brand, Ottawa
Taylor BR, Parkinson D, Parsons WFJ (1989) Nitrogen and lignin content as predictors of litter decay rates: a microcosm test. Ecology 70:97–104
Thibodeau L, Raymond P, Camire C, Munson AD (2000) Impact of precommercial thinning in balsam fir stands on soil nitrogen dynamics, microbial biomass, decomposition, and foliar nutrition. Can J For Res 30:229–238
Trofymow JA, Preston CM, Prescott CE (1995) Litter quality and its potential effect on decay rates of materials from Canadian forests. Water Air Soil Pollut 82:215–226
Vitousek PM (1998) Foliar and litter nutrients, nutrient resorption, and decomposition in Hawaiian Metrosideros polymorpha. Ecosystems 1:401–407
Yamasaki SH, Fyles JW, Egger KN, Titus BD (1998) The effect of Kalmia angustifolia on the growth, nutrition, and ectomycorrhizal symbiont community of black spruce. For Ecol Manage 105:197–207
Acknowledgements
We are grateful to D. Gasser, B. Lapointe, M. Lavoie, J. Lamontagne, B. Lafleur and L-M. Thériault for technical assistance, as well as to one anonymous reviewer for his valued comments. We thank the DOMTAR Paper Products company for authorising access to the research site from which soil was collected. The study was funded by research grants awarded to the corresponding author by the Ministère des ressources naturelles du Québec–Fonds forestier, the Natural Sciences and Engineering Research Council of Canada (NSERC) and the Fonds pour la Formation de chercheurs et aide à la recherche (FCAR).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sommerville, D., Bradley, R. & Mailly, D. Leaf litter quality and decomposition rates of yellow birch and sugar maple seedlings grown in mono-culture and mixed-culture pots at three soil fertility levels. Trees 18, 608–613 (2004). https://doi.org/10.1007/s00468-004-0354-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-004-0354-7