Abstract
A study was made, in a cool-temperate zone, of the extent of cell division in the cambium, the extent of differentiation of cambial derivatives, and the localization of storage starch around the cambium in locally heated (22–26°C) stems of the evergreen conifer Abies sachalinensis (Schmidt) Masters during cambial dormancy and immediately after natural reactivation of the cambium. In locally heated regions of stems during cambial dormancy, heating induced localized reactivation of the cambium. However, the cells in the heated and reactivated cambium stopped dividing soon after only a few cells had been generated. In addition, no differentiation of the xylem and the disappearance of starch from storage tissues around the cambium were observed. In regions of stem that had been locally heated after natural reactivation of the cambium, cell division continued in the cambium and earlywood tracheids with a large radial diameter and secondary walls were formed, with abundant starch in the storage tissues around the cambium. Our results suggest that the extent of both cell division in the cambium and cell differentiation depends on the amount of starch in storage tissues around the cambium in the locally heated stems of an evergreen conifer growing in a cool-temperate zone.
Similar content being viewed by others
Introduction
The cambial activity of coniferous trees in temperate zones exhibits periodicity, which is characterized by the formation of earlywood and latewood and winter dormancy. The quality and quantity of the wood and the bark of trees reflect the periodicity. Therefore, many researchers have been interested in the events associated with cambial activity and have studied the anatomical, biochemical, cytological and histochemical changes that occur in the cambium (e.g. Catesson 1994; Larson 1994).
Efforts have been made to estimate the extent of cambial dormancy and to identify factors that maintain and/or break dormancy. For example, cambial growth has been monitored in shoot and stem cuttings that have been exposed to controlled conditions that are favorable for cambial growth (Little and Bonga 1974; Riding and Little 1984, 1986; Sundberg et al. 1987; Mellerowicz et al. 1992) and in locally heated regions of intact stems (Savidge and Wareing 1981; Barnett and Miller 1994; Oribe and Kubo 1997; Oribe et al. 2001) during cambial dormancy. The resultant observations in various evergreen conifers indicate that winter cambial dormancy consists of a resting stage, during which dormancy is maintained by internal agents or conditions, and a quiescent stage, during which dormancy is imposed by external factors (Little and Bonga 1974). During the quiescent stage of cambial dormancy, the cambium has the potential for the generation of cells, and this potential is realized whenever certain temperature conditions are satisfied (Savidge and Wareing 1981; Barnett and Miller 1994; Oribe and Kubo 1997).
In a previous study, we showed that localized heating of stems for 5 or 6 days induced cambial reactivation and we repeatedly monitored the series of events that occurred in stems of the evergreen conifer Abies sachalinensis (Schmidt) Masters during cambial reactivation (Oribe et al. 2001). However, 2-week localized heating of intact stems during cambial dormancy revealed some variations in the extent of cambial activity and xylem differentiation in heated regions that depended on the season and on the position of the heated region of the stem in the evergreen conifer Cryptomeria japonica (L. f.) D. Don (Oribe and Kubo 1997). Such observations indicate that factors or conditions in addition to an increase in temperature are involved in the extent of cambial growth after cambial reactivation in evergreen conifers.
The present study was designed to identify the factors or conditions that regulate the extent of cell division in the cambium and of differentiation of cambial derivatives after cambial reactivation in an evergreen conifer. In woody plants, storage starch is an important source of many kinds of organic compound, including sucrose, which is the primary sugar that is transported in plants (Shiroya et al. 1962; Zimmermann 1971; Giaquinta 1980; Kozlowski and Pallardy 1997) and regulates vascular differentiation in cultured cells and tissues (Wetmore and Rier 1963; Warren Wilson et al. 1994). Accordingly, during the quiescent stage of dormancy of the cambium and also immediately after natural reactivation of the cambium, we monitored the extent of cell division in the cambium, the extent of differentiation of cambial derivatives, and the localization of storage starch around the cambium in locally heated portions of stems of A. sachalinensis. Localized heating of evergreen conifers seems to provide a useful experimental model for studies of the dynamics of cambial reactivation in intact trees (Oribe et al. 2001).
Materials and methods
Plant material
The plant material examined in this study comprised 42-year-old specimens of Abies sachalinensis (Schmidt) Masters (average height, 13 m; average diameter at breast height, 32 cm) that belonged to the clone "Shimokawa 125". Three trees in the seed orchard of the Hokkaido Regional Breeding Office (43°04′N, 141°31′E) were selected for analysis.
Heat treatment
Localized heat treatments were applied from 10 to 25 March 1998 (the first experiment), from 20 to 27 April 1998 (the second experiment) and from 22 February to 15 March 1999 (the third experiment), as described in previous reports (Oribe and Kubo 1997; Oribe et al. 2001). One or two pliable electric heating tapes (Silicone-Rubber Heater; O & M Heater, Nagoya, Japan), 30 cm in length and 50 cm in width, were wrapped around the main stem of each tree at breast height. An alternating current of 100 V was passed through each tape to heat the surface of the stem. The temperature between the outer bark and the heating tape was adjusted to 22–26°C with a thermostat and was recorded with a thermocouple during each experiment.
Preparation of samples for light microscopy
Samples were removed from the heated area of each tree daily during the first and second experiments and weekly during the third experiment. A block (2×2 cm2) containing the entire rhytidome, phloem, cambium and some xylem was removed with disposable scalpels and a chisel. Each block was trimmed to 1-cm cubes and 2-mm-thick cuttings immediately after sampling.
The 1-cm cubes were fixed in 2.5% glutaraldehyde plus 3.7% formaldehyde in 0.05 M phosphate buffer (pH 7.2) for 3 days, and cubes were then washed in running water. The fixed cubes were dehydrated by immersion in a graded ethanol series and diethyl ether and then embedded in celloidin. Transverse and radial sections were cut at thicknesses of 20–30 μm on a sliding microtome from each embedded sample.
The 2-mm-thick cuttings were fixed in 5% glutaraldehyde in 0.05 M phosphate buffer (pH 7.2) for 15 min at room temperature under a vacuum. Only those samples that had been seen to sink were then incubated overnight in fresh fixation medium (5% glutaraldehyde in 0.05 M phosphate buffer) at room temperature. Fixed cuttings were washed in 0.1 M phosphate buffer and trimmed to 3 mm in length for subsequent fixation in 1% osmium tetroxide in 0.05 M phosphate buffer (pH 7.2) for 6 h at room temperature. After washing in phosphate buffer, the specimens were dehydrated in a graded ethanol series and embedded in Spurr's resin (Spurr 1969). Transverse and radial sections were cut at a thickness of approximately 1 μm with glass knives on an ultramicrotome (Ultracut J; Reichert, Vienna, Austria).
Sections for observations of storage starch were stained with a solution of iodine-potassium iodide in water, and other sections were stained with a solution of 0.2% azure B in water or a solution of 0.5% safranin in 50% ethanol. Some sections were not stained. The sections were examined under a light microscope (BX50-BF; Olympus Optical, Tokyo, Japan) or a Nomarski differential interference contrast microscope (BX50-DIC; Olympus Optical, Tokyo, Japan).
Anatomical characterization of cells in cambial regions
In A. sachalinensis, dormant cambium was directly adjacent to narrow-diameter and thick-walled latewood tracheids that had formed at the end of the previous growing season and the previous year's sieve cells that had been crushed to some extent (Fig. 1A). After cambial reactivation, namely, the re-initiation of cell division in the cambium, newly formed xylem cells with thin cell walls were observed between the previous year's latewood tracheids and the cambium. Current phloem cells were accumulated between the cambium and the crushed sieve cells. In Larix kaempferi (Lamb.) Carrière, Imagawa (1981) monitored the seasonal development of phloem by reference to such crushed sieve cells. According to the anatomical criteria described below, we chose cells between the previous year's latewood tracheids and the crushed sieve cells for measurements of the extent of cambial growth (Fig. 2A). Both xylem and phloem cells with secondary walls were identified by the birefringence within cell walls under the Nomarski differential interference contrast microscope (Riding and Little 1984). Cells for which the ratio of the radial diameter to the tangential diameter did not exceed 0.5, which was the minimum value of the ratio of the radial diameter to the tangential diameter of a pair of daughter cells that had recently been generated, were defined as cambial cells. Cambial derivatives between the cambium and a zone of cells with secondary walls were considered to be cells at the radial-enlargement stage of differentiation. The average number of each type of cell was calculated for ten radial files in all samples collected during heat treatments. We did not examine a sample that had been heated for 13 days, collected on 23 March 1998, during the first experiment because some damaged tissues had formed in the cambial region. No similarly damaged tissues were observed in other samples.
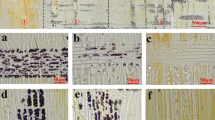
Localization of storage starch (arrows) around the cambium in a series of non-heated and heated samples as viewed under a Nomarski differential interference contrast microscope (A) and a light microscope (B–D). A A transverse section of a non-heated sample collected on 22 February 1999. The previous year's sieve cells that had been crushed to some extent (arrowheads) are visible. Bar 30 μm. B A transverse section of a 15-day-heated sample collected on 25 March 1998. Bar 30 μm. C A radial section of a non-heated sample collected on 20 April 1998. Bar 100 μm. D A radial section of a 7-day-heated sample collected on 27 April 1998. Bar 100 μm. (ex Radially enlarging xylem cells, fc fusiform cambial cells, pc procumbent ray cambial cells, pp phloem parenchyma cells, r ray cells, xy xylem)
The extent of cambial growth in a series of heated samples as viewed transversally under a light microscope. A A 7-day-heated sample collected on 27 April 1998. Phloem cells with secondary walls (ps), radially enlarging phloem cells (ep), fusiform cambial cells (fc), radially enlarging xylem cells (ex) and xylem cells with secondary walls (xs) can be seen. Bar 60 μm. B A non-heated sample collected on 20 April 1998. Newly formed cell walls (arrowheads) can be seen in the cambium. Bar 30 μm. C A 21-day-heated sample collected on 15 March 1999. Some fusiform cambial cells show signs of autolysis (arrowheads). Bar 30 μm. The bottom of each photograph corresponds to the xylem (xy) side. Arrows indicate the previous year's sieve cells that had been crushed to some extent. pp Phloem parenchyma cells, r ray cells
Results
Climate during the experiments
The air temperature during the experiments, as recorded at Nishi-Nopporo, which is located 14 km from the Hokkaido Regional Breeding Office, is shown in Fig. 3. The site is classified as being in a cool-temperate zone. The maximum temperature was below 8°C and the minimum temperature was below freezing during the first experiment (from 10 to 25 March 1998) and the third experiment (from 22 February to 15 March 1999). The temperature never fell below freezing and was sometimes above 20°C during the second experiment (from 20 to 27 April 1998). Thus, air temperatures during the first and third experiments were much lower than those during the second experiment.
Cambial activity at the beginning of each experiment
In non-heated samples, collected on 10 March 1998 and on 22 February 1999 when the first and third experiments were started, there were no dividing cambial cells, an indication that the cambium was dormant in both cases (Fig. 1A). The cambium had already resumed cell division naturally and some new cambial derivatives had been formed in a non-heated sample that was collected on 20 April 1998, when the second experiment was started (Fig. 2B). Thus, localized heat treatments were applied to the dormant cambium during the first and third experiments, while active cambium was heated during the second experiment.
The extent of cambial growth in locally heated stems
The numbers of cells between the crushed sieve cells and the previous year's latewood tracheids in samples collected during the various experiments are shown in Fig. 4. In a non-heated sample, collected on 10 March 1998, when the first experiment was started, the cells between the crushed sieve cells and the previous year's latewood tracheids consisted of five dormant cambial cells. We observed no visible cytological changes related to the re-initiation of neither cell division of fusiform cambial cells nor any xylem differentiation in samples that had been heated for 1–5 days and were collected between 11 and 15 March 1998. However, radial enlargement and deposition of secondary walls of differentiating phloem had occurred. Fusiform cambial cells resumed cell division in samples, collected on 16 March 1998, which had been heated for 6 days.
The numbers of phloem cells (open triangles), cambial cells (open circles) and xylem cells (open squares) between the previous year's latewood tracheids and the crushed sieve cells, and the total number of cells (filled circles) in samples that were collected during the first (A), second (B) and third (C) experiments. Vertical bars indicate the standard errors
The number of cells between the crushed sieve cells and the previous year's latewood tracheids in a sample, collected on 18 March 1998, that had been heated for 8 days during the first experiment, was almost the same as that in a non-heated sample collected on 20 April 1998, when the second experiment was started (Fig. 4A, B). Cells between the crushed sieve cells and the previous year's latewood tracheids consisted of one phloem cell with a secondary wall, one radially enlarging phloem cell, seven cambial cells and one radially enlarging xylem cell in the 8-day-heated sample. The cells consisted of two radially enlarging phloem cells, seven cambial cells and one radially enlarging xylem cell in the non-heated sample. These observations indicated that the extent of cambial growth in both samples was almost identical.
We compared the extent of cell division in the cambium and the extent of differentiation of cambial derivatives in samples that had been heated for 8–15 days, collected between 18 and 25 March 1998 during the first experiment, with those in samples that were collected between 20 and 27 April 1998 during the second experiment. We postulated that such a comparison would reveal the effects of 7-day localized heat treatment on cambial growth in heated reactivated cambium and in naturally reactivated cambium. The heated cambium produced four cells during the period from 18 to 25 March 1998 and 17 cells during the second experiment (Fig. 4A, B). Thus, the effect of the localized heat treatment on the extent of cell division in the cambium was more conspicuous in the naturally reactivated cambium than in the heated reactivated cambium. There were approximately three phloem cells and this number remained almost constant during the heat treatment but the number of phloem cells with secondary walls increased gradually in heated samples that were collected both between 18 and 25 March 1998 during the first experiment and during the second experiment (Fig. 4A, B). In samples that had been heated for 8–15 days during the first experiment, we observed only a few radially enlarging xylem cells (Fig. 4A). In addition, the radial diameters of these cells, which ranged from 8 to 15 μm (Fig. 1B), were markedly smaller than the typical diameters of earlywood tracheids of A. sachalinensis, which range from 30 to 60 μm (Wood Technological Association of Japan 1989). By contrast, during the second experiment, 12 xylem cells were produced (Fig. 4B) and a few earlywood tracheids with a large radial diameter and secondary walls were observed in a 7-day-heated sample that was collected on 27 April 1998 (Fig. 2A).
The cells between the crushed sieve cells and the previous year's latewood tracheids consisted of six cambial cells in a non-heated sample that was collected on 22 February 1999 when the third experiment was started (Figs. 1A, 4C). In samples that had been heated for 8 days, collected on 2 March 1999 during the third experiment, fusiform cambial cells had resumed cell division and three radially enlarging phloem cells were observed. The number of cells between the crushed sieve cells and the previous year's latewood tracheids barely changed in samples, collected between 2 and 15 March 1999, that had been heated for 8–21 days. In a sample that had been heated for 21 days, collected on 15 March 1999, we observed no mitotic figures and no newly divided fusiform cambial cells, an indication that cell division in the cambium had ceased by the end of the third experiment. In addition, fusiform cambial cells showed signs of autolysis (Fig. 2C). In samples that had been heated for 8–21 days, there were approximately three phloem cells and this number was almost constant but the number of phloem cells with secondary walls increased gradually, as noted above in the case of heated samples that were collected during the first and second experiments (Fig. 4). No differentiating xylem cells were observed in heated samples collected during the third experiment (Fig. 2C).
Localization of storage starch around the cambium in locally heated stems
During cambial dormancy, storage starch accumulated in the phloem parenchyma cells and in the ray cells nearest the cambium, but no storage starch was evident in fusiform cambial cells (Fig. 1A). In samples that had been heated for 1–15 days during the first experiment and for 8–14 days during the third experiment, the amount of storage starch decreased in the phloem parenchyma cells and in the ray cells nearest the cambium, while the amount of storage starch increased in fusiform cambial cells during heat treatments (Fig. 1B). No storage starch was evident in the phloem parenchyma cells, in the ray cells nearest the cambium, and in fusiform cambial cells in the 21-day-heated sample that was collected on 15 March during the third experiment.
In the non-heated sample collected on 20 April 1998, when the second experiment was started, no storage starch was observed in fusiform cambial cells (Fig. 1C). The amount of storage starch increased in fusiform cambial cells in heated samples collected during the second experiment. In all samples collected during the second experiment, storage starch was abundant in the phloem parenchyma cells and in the ray cells nearest the cambium (Fig. 1C, D).
Discussion
Localized heating of stems of A. sachalinensis during late winter induced the localized reactivation of the cambium. This observation is in agreement with previous observations of cambial reactivation in locally heated stems of several evergreen conifers in winter (Savidge and Wareing 1981; Barnett and Miller 1994; Oribe and Kubo 1997; Oribe et al. 2001). It is clear, therefore, that cambial cells of stems at the quiescent stage of cambial dormancy, which is imposed by external factors such as low temperature (Little and Bonga 1974), can re-initiate cell division independently of the growth of new shoots and the development of buds in spring. However, the effect of localized heating on the extent of cell division in heated reactivated cambium was not as conspicuous as that in naturally reactivated cambium. In addition, heated reactivated cambium ceased cell division soon after a few cells had been produced (Figs. 2C, 4). Our observations suggest that, in A. sachalinensis, continuous cell division in heated reactivated cambium requires additional conditions, which appear to be satisfied in naturally reactivated cambium.
The cambium must be a strong sink for sucrose (Krabel 2000), which is the primary photosynthate that is transported within the source-sink system in plants (Shiroya et al. 1962; Zimmermann 1971; Giaquinta 1980; Kozlowski and Pallardy 1997). Some of the transported sucrose is stored as starch in storage tissues, for example, the ray parenchyma cells and phloem parenchyma cells (Zimmermann 1971; Blechschmidt-Schneider 1990) in stems. In heated stems of A. sachalinensis, we previously observed the disappearance of starch from procumbent ray cambial cells and phloem parenchyma cells, as well as the accumulation of starch in fusiform cambial cells, during cambial reactivation (Oribe et al. 2001). Moreover, 14C-labeled assimilate was translocated radially towards the cortex and the pith in branches of Picea abies (L.) Karsten that had been warmed in winter (Blechschmidt-Schneider 1990). These observations suggest the influx of sucrose, derived from starch in storage tissues, into the cambium. The present study demonstrated that cells of heated reactivated cambium stopped dividing with the disappearance of starch from storage tissues around the cambium, and that cells in the naturally reactivated cambium continued to divide with the concomitant abundance of starch in the storage tissues. Therefore, the extent of cell division in the cambium appeared to depend on the sucrose supplied by the storage tissue to the cambium. The cells in the heated reactivated cambium might cease to divide as a result of the absence of a supply of sucrose. Thus, we can hypothesize that the continuation of cell division in the cambium after cambial reactivation requires a continuous supply of sucrose.
Sucrose is derived from photosynthates, which are produced in needles, and it moves basipetally to storage tissues through the phloem. During the winter, endogenous [14C]-sucrose and 14C-labeled assimilate were transported basipetally in stems of Pinus contorta Douglas ex Loud. var. latifolia Engelm. et S. Watson (Savidge and Wareing 1982) and in warmed branches of Picea abies (Blechschmidt-Schneider 1990). These observations indicate that the translocation of sucrose through the phloem might occur in evergreen conifers in winter. Photosynthesis does occur at lower temperatures, even though respiration overtakes photosynthesis at several degrees below freezing (e.g. Kramer and Kozlowski 1960). Therefore, winter suppression of net photosynthesis, which is the difference between actual photosynthesis and respiration, occurs in evergreen conifers under cold winter conditions. Net photosynthesis has been observed in some evergreen conifers under mild-temperate winter conditions (Fry and Phillip 1977). However, net photosynthesis was close to zero or sometimes negative in an evergreen conifer under severe cold-winter conditions (Troeng and Linder 1982). In A. sachalinensis in a cool-temperate zone, the winter suppression of photosynthesis, caused by low air temperatures with minimum temperatures below freezing (Fig. 3), might limit the supply of sucrose to storage tissues around the cambium. Consequently, starch would disappear from storage tissues around the cambium in locally heated regions of stems in winter.
It is likely that other endogenous compounds in addition to sucrose regulate cambial growth. It has been proposed that indole-3-acetic acid (IAA), an auxin, is required for the maintenance of meristematic activity and for the elongated shape of fusiform cambial cells and the radial enlargement of cambial derivatives (Savidge and Wareing 1981; Savidge 1983; Sundberg et al. 2000). The basipetal transport of endogenous [14C]-IAA in shoots or stems (Little 1981; Savidge and Wareing 1982; Odani 1985) and the relatively high levels of endogenous IAA in the cambium (Savidge and Wareing 1982; Sundberg et al. 1991; Funada et al. 2001, 2002) during cambial dormancy suggest the possibility that IAA might be supplied to the cambium in winter. However, in the heated reactivated cambium of A. sachalinensis in a cool-temperate zone, active division of cells and maintenance of the morphological state of newly formed fusiform cambial cells might require more IAA than is supplied normally during the winter. The failure of cambial derivatives to differentiate to xylem, in addition to the early cessation of cell division, might be a result of IAA deficiency in the cambium in locally heated stems during cambial dormancy. In naturally reactivated cambium, a continuous supply of IAA from elongating shoots and/or expanding buds can maintain active cambial division and the subsequent differentiation of xylem.
In locally heated regions of stems of A. sachalinensis during cambial dormancy, a few fusiform cambial cells on the phloem side of the cambium differentiated into phloem cells prior to the re-initiation of cell division in the cambium (Fig. 4A), and only minimal differentiation of xylem occurred after cambial reactivation. This result is in agreement with results of previous studies, in which phloem differentiation was observed prior to xylem differentiation in conifers (Wilson 1966; Alfieri and Evert 1968, 1973). Imagawa (1981) observed that some fusiform cambial cells on the phloem side of the cambium, which had been produced at the end of the previous growing season, overwintered in an immature state and began to differentiate into phloem cells prior to the reactivation of cambium in an intact deciduous conifer, L. kaempferi. Such overwintered cells have also been observed in other species (Alfieri and Evert 1968, 1973; Esau 1969), including A. balsamea (L.) Miller (Kutscha et al. 1975; Riding and Little 1984), which belongs to the same genus as A. sachalinensis. We can, therefore, hypothesize that newly formed phloem is necessary for the re-initiation of cell division in the cambium or for the formation of xylem in conifers. Overwintered cells can initiate the formation of phloem prior to cambial reactivation and differentiation of the xylem.
Localized heating of the stem of A. sachalinensis in a cool-temperate zone failed to induce xylem differentiation during cambial dormancy (Figs. 2C, 4A, C). However, we observed previously that localized heating of stems of C. japonica in a temperate zone induced the formation of earlywood tracheids with a large radial diameter and secondary walls during cambial dormancy (Oribe and Kubo 1997). Localized heating of stems affects the extent of the differentiation to xylem of cambial derivatives, depending on climatic conditions at the experimental site. There were differences in air temperature between our present and previous experiments. Air temperatures recorded in late winter in Hokkaido, a cool-temperate zone, were much lower than those in winter in a temperate zone (Fig. 3; Oribe and Kubo 1997). Thus, the extent of xylem differentiation in locally heated regions of stems of evergreen conifers could be species specific, but might also depend on air temperatures during the experiments.
References
Alfieri FJ, Evert RF (1968) Seasonal development of the secondary phloem in Pinus. Am J Bot 55:518–528
Alfieri FJ, Evert RF (1973) Structure and seasonal development of the secondary phloem in the Pinaceae. Bot Gaz 134:17–25
Barnett JR, Miller H (1994) The effect of applied heat on graft union formation in dormant Picea sitchensis (Bong.) Carr. J Exp Bot 45:135–143
Blechschmidt-Schneider S (1990) Phloem transport in Picea abies (L.) Karst. in mid-winter. I Microautoradiographic studies on 14C-assimilate translocation in shoots. Trees 4:179–186
Catesson AM (1994) Cambial ultrastructure and biochemistry: changes in relation to vascular tissue differentiation and the seasonal cycle. Int J Plant Sci 155:251–261
Esau K (1969) The phloem. Borntraeger, Berlin
Fry DJ, Phillips IDJ (1977) Photosynthesis of conifers in relation to annual growth cycles and dry matter production. II. Seasonal photosynthetic capacity and mesophyll ultrastructure in Abies grandis, Picea sitchensis, Tsuga heterophylla and Larix leptolepis growing in S.W. England. Physiol Plant 40:300–306
Funada R, Kubo T, Tabuchi M, Sugiyama T, Fushitani M (2001) Seasonal variations in endogenous indole-3-acetic acid and abscisic acid in the cambial region of Pinus densiflora stems in relation to earlywood-latewood transition and cessation of tracheid production. Holzforschung 55:128–134
Funada R, Kubo T, Sugiyama T, Fushitani M (2002) Changes in levels of endogenous plant hormones in cambial regions of stems of Larix kaempferi at the onset of cambial activity in springtime. J Wood Sci 48:75–80
Giaquinta RT (1980) Translocation of sucrose and oligosaccharides. In: Preiss J (ed) The biochemistry of plants, vol 3. Academic Press, New York, pp 271–320
Imagawa H (1981) Study on the seasonal development of the secondary phloem in Larix leptolepis. Res Bull Exp For Hokkaido Univ 38:31–44
Kozlowski TT, Pallardy SG (1997) Physiology of woody plants, 2nd edn. Academic Press, San Diego
Krabel D (2000) Influence of sucrose on cambial activity. In: Savidge RA, Barnett JR, Napier R (eds) Cell and molecular biology of wood formation. BIOS Scientific, Oxford, pp 113–125
Kramer PJ, Kozlowski TT (1960) Physiology of trees. McGraw-Hill, New York
Kutscha NP, Hyland F, Schwarzmann JM (1975) Certain seasonal changes in balsam fir cambium and its derivatives. Wood Sci Technol 9:175–188
Larson PR (1994) The vascular cambium: development and structure. Springer, Berlin Heidelberg New York
Little CHA (1981) Effect of cambial dormancy state on the transport of [1-14C]indol-3-ylacetic acid in Abies balsamea shoots. Can J Bot 59:342–348
Little CHA, Bonga JM (1974) Rest in the cambium of Abies balsamea. Can J Bot 52:1723–1730
Mellerowicz EJ, Coleman WK, Riding RT, Little CHA (1992) Periodicity of cambial activity in Abies balsamea. I. Effects of temperature and photoperiod on cambial dormancy and frost hardiness. Physiol Plant 85:515–525
Odani K (1985) Indole-3-acetic acid transport in pine shoots under the stage of true dormancy. J Jpn For Soc 67:332–334
Oribe Y, Kubo T (1997) Effect of heat on cambial reactivation during winter dormancy in evergreen and deciduous conifers. Tree Physiol 17:81–87
Oribe Y, Funada R, Shibagaki M, Kubo T (2001) Cambial reactivation in locally heated stems of the evergreen conifer Abies sachalinensis (Schmidt) Masters. Planta 212:684–691
Riding RT, Little CHA (1984) Anatomy and histochemistry of Abies balsamea cambial zone cells during the onset and breaking of dormancy. Can J Bot 62:2570–2579
Riding RT, Little CHA (1986) Histochemistry of the dormant vascular cambium of Abies balsamea: changes associated with tree age and crown position. Can J Bot 64:2082–2087
Savidge RA (1983) The role of plant hormones in higher plant cellular differentiation. II. Experiments with the vascular cambium, and sclereid and tracheid differentiation in the pine, Pinus contorta. Histochem J 15:447–466
Savidge RA, Wareing PF (1981) Plant-growth regulators and the differentiation of vascular elements. In: Barnett JR (ed) Xylem cell development. Castle House, London, pp 192–235
Savidge RA, Wareing PF (1982) Apparent auxin production and transport during winter in the nongrowing pine tree. Can J Bot 60:681–691
Shiroya T, Lister GR, Slankis V, Krotkov G, Nelson CD (1962) Translocation of the products of photosynthesis to roots of pine seedlings. Can J Bot 40:1125–1135
Spurr AR (1969) A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res 26:31–43
Sundberg B, Little CHA, Riding RT, Sandberg G (1987) Levels of endogenous indole-3-acetic acid in the vascular cambium region of Abies balsamea trees during the activity—rest—quiescence transition. Physiol Plant 71:163–170
Sundberg B, Little CHA, Cui K, Sandberg G (1991) Level of endogenous indole-3-acetic acid in the stem of Pinus sylvestris in relation to the seasonal variation of cambial activity. Plant Cell Environ 14:241–246
Sundberg B, Uggla C, Tuominen H (2000) Cambial growth and auxin gradients. In: Savidge RA, Barnett JR, Napier R (eds) Cell and molecular biology of wood formation. BIOS Scientific, Oxford, pp 169–188
Troeng E, Linder S (1982) Gas exchange in a 20-year-old stand of Scots pine. I. Net photosynthesis of current and one-year-old shoots within and between seasons. Physiol Plant 54:7–14
Warren Wilson J, Roberts LW, Warren Wilson PM, Gesshoff PM (1994) Stimulatory and inhibitory effects of sucrose concentration on xylogenesis in lettuce pith explants; possible mediation by ethylene biosynthesis. Ann Bot 73:65–73
Wetmore RH, Rier JP (1963) Experimental induction of vascular tissues in callus of angiosperms. Am J Bot 50:418–430
Wilson BF (1966) Mitotic activity in the cambial zone of Pinus strobus. Am J Bot 53: 364–372
Wood Technological Association of Japan (1989) Todomatsu. In: Wood Technological Association of Japan (ed) Nippon no mokuzai, 3rd edn (in Japanese). Wood Technological Association of Japan, Tokyo, pp 10–11
Zimmermann MH (1971) Storage, mobilization and circulation of assimilates. In: Zimmermann MH, Brown CL (eds) Trees: structure and function. Springer, Berlin Heidelberg New York, pp 307–322
Acknowledgements
The research was supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (no. JSPS-RFTF 96L00605).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oribe, Y., Funada, R. & Kubo, T. Relationships between cambial activity, cell differentiation and the localization of starch in storage tissues around the cambium in locally heated stems of Abies sachalinensis (Schmidt) Masters. Trees 17, 185–192 (2003). https://doi.org/10.1007/s00468-002-0231-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-002-0231-1