Abstract
Background
Steroid-resistant nephrotic syndrome (SRNS) is a major cause of stage 5 chronic kidney disease (CKD 5) in children. LDL apheresis (LDL-A) is now FDA approved for the treatment of pediatric focal segmental glomerulosclerosis (FSGS). Effective management of hyperlipidemia with LDL-A in SRNS patients may prevent progression of kidney disease and lead to remission. We report a case series of patients who received LDL-A for treatment of SRNS
Methods
We describe five children with SRNS who were treated with 12 sessions of LDL-A. Partial remission (PR) is defined as urine protein to creatinine ratio (UPC) of 0.2–2 (g/g) or decrease in UPC ≥ 50%, and complete remission (CR) is defined as UPC < 0.2 (g/g).
Results
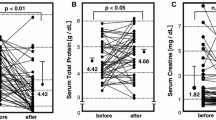
One patient achieved CR and three achieved PR. One patient did not respond to therapy. The earliest that a patient achieved PR was at treatment #10 and some did not respond until after LDL-A was completed. Those who responded stayed in either CR or PR for extended periods of time. LDL-A was successful at significantly reducing LDL (p < 0.001), total cholesterol (p < 0.001), and triglyceride (p < 0.001).
Conclusions
LDL-A was able to significantly decrease the lipid levels in these patients and induce CR and PR in the majority. The current study confirms previous studies showing those with a higher glomerular sclerosis burden were less likely to respond. LDL-A should be considered in patients with treatment-resistant SRNS and should be considered before there is a high burden of glomerular sclerosis to provide the best chance of success.



Similar content being viewed by others
Data availability
The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Nourbakhsh N, Mak RH (2017) Steroid-resistant nephrotic syndrome: past and current perspectives. Pediatr Health Med Ther 8:29–37
(1982) Early identification of frequent relapsers among children with minimal change nephrotic syndrome. A report of the International Study of Kidney Disease in Children. J Pediatr 101:514–518
Tarshish P, Tobin JN, Bernstein J, Edelmann CM Jr (1997) Prognostic significance of the early course of minimal change nephrotic syndrome: report of the International Study of Kidney Disease in Children. J Am Soc Nephrol 8:769–776
Mekahli D, Liutkus A, Ranchin B, Yu A, Bessenay L, Girardin E, Van Damme-Lombaerts R, Palcoux JB, Cachat F, Lavocat MP, Bourdat-Michel G, Nobili F, Cochat P (2009) Long-term outcome of idiopathic steroid-resistant nephrotic syndrome: a multicenter study. Pediatr Nephrol 24:1525–1532
Saran R, Robinson B, Abbott KC, Agodoa LYC et al (2019) US Renal Data System 2018 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis 73(3 Suppl 1):A7–A8
Liposorber LA-15 system - FDA Humanitarian Device Exemption. Apheresis For Focal Glomeruloscleprosis In Adults and Pediatric Patients. HDE H120005. 3/20/2018
Moorhead JF, Chan MK, El-Nahas M, Varghese Z (1982) Lipid nephrotoxicity in chronic progressive glomerular and tubulo-interstitial disease. Lancet 2:1309–1311
Keane WF, Kasiske BL, O’Donnell MP, Kim Y (1991) The role of altered lipid metabolism in the progression of renal disease: experimental evidence. Am J Kidney Dis 17(5 Suppl 1):38–42
Keane WF, Mulcahy WS, Kasiske BL, Kim Y, O’Donnell MP (1991) Hyperlipidemia and progressive renal disease. Kidney Int Suppl 31:S41-48
Hattori M, Chikamoto H, Akioka Y, Nakakura H, Ogino D, Matsunaga A, Fukazawa A, Miyakawa S, Khono M, Kawaguchi H, Ito K (2003) A combined low-density lipoprotein apheresis and prednisone therapy for steroid-resistant primary focal segmental glomerulosclerosis in children. Am J Kidney Dis 42:1121–1130
Shah L, Hooper DK, Okamura D, Wallace D, Moodalbail D, Gluck C, Koziell A, Zaritsky JJ (2019) LDL-apheresis-induced remission of focal segmental glomerulosclerosis recurrence in pediatric renal transplant recipients. Pediatr Nephrol 34:2343–2350
Kawasaki Y, Suzuki S, Matsumoto A, Takano K, Suyama K, Hashimoto K, Suzuki J, Suzuki H, Hosoya M (2007) Long-term efficacy of low-density lipoprotein apheresis for focal and segmental glomerulosclerosis. Pediatr Nephrol 22:889–892
Oto J, Suga K, Matsuura S, Kondo S, Ohnishi Y, Inui D, Imanaka H, Kagami S, Nishimura M (2009) Low-density lipoprotein apheresis in a pediatric patient with refractory nephrotic syndrome due to focal segmental glomerulosclerosis. J Anesth 23:284–287
Hattori M, Ito K, Kawaguchi H, Tanaka T, Kubota R, Khono M (1993) Treatment with a combination of low-density lipoprotein aphaeresis and pravastatin of a patient with drug-resistant nephrotic syndrome due to focal segmental glomerulosclerosis. Pediatr Nephrol 7:196–198
Kobayashi S (2008) Applications of LDL-apheresis in nephrology. Clin Exp Nephrol 12:9–15
Athyros VG, Mikhailidis DP, Papageorgiou AA, Symeonidis AN, Pehlivanidis AN, Bouloukos VI, Elisaf M (2004) The effect of statins versus untreated dyslipidaemia on renal function in patients with coronary heart disease. A subgroup analysis of the Greek atorvastatin and coronary heart disease evaluation (GREACE) study. J Clin Pathol 57:728–734
Sandhu S, Wiebe N, Fried LF, Tonelli M (2006) Statins for improving renal outcomes: a meta-analysis. J Am Soc Nephrol 17:2006–2016
Bussolati B, Deregibus MC, Fonsato V, Doublier S, Spatola T, Procida S, Di Carlo F, Camussi G (2005) Statins prevent oxidized LDL-induced injury of glomerular podocytes by activating the phosphatidylinositol 3-kinase/AKT-signaling pathway. J Am Soc Nephrol 16:1936–1947
Kong X, Yuan H, Fan J, Li Z, Wu T, Jiang L (2013) Lipid-lowering agents for nephrotic syndrome. Cochrane Database Syst Rev (12):Cd005425. https://doi.org/10.1002/14651858.CD005425.pub2
Lupien P-J, Moorjani S, Awad J (1976) A new approach to the management of familial hypercholesterolaemia: removal of plasma-cholesterol based on the principle of affinity chromatography. Lancet 1:1261–1265
Tojo K, Sakai S, Miyahara T (1988) Possible therapeutic application of low density lipoprotein apheresis (LDL-A) in conjunction with double filtration plasmapheresis (DFPP) in drug-resistant nephrotic syndrome due to focal glomerular sclerosis (FGS). Nihon Jinzo Gakkai Shi 30:1153–1160
Tojo K, Sakai S, Miyahara T (1990) Therapeutic trial of low density lipoprotein apheresis (LDL-A) in conjunction with double filtration plasmapheresis (DFPP) in drug-resistant nephrotic syndrome due to focal glomerular sclerosis (FGS). Prog Clin Biol Res 337:193–194
Muso E, Yashiro M, Matsushima M, Yoshida H, Sawanishi K, Sasayama S (1994) Does LDL-apheresis in steroid-resistant nephrotic syndrome affect prognosis? Nephrol Dial Transplant 9:257–264
Muso E, Mune M, Hirano T, Hattori M, Kimura K, Watanabe T, Yokoyama H, Sato H, Uchida S, Wada T, Shoji T, Yuzawa Y, Takemura T, Sugiyama S, Nishizawa Y, Ogahara S, Yorioka N, Sakai S, Ogura Y, Yukawa S, Iino Y, Imai E, Matsuo S, Saito T (2015) Immediate therapeutic efficacy of low-density lipoprotein apheresis for drug-resistant nephrotic syndrome: evidence from the short-term results from the POLARIS Study. Clin Exp Nephrol 19:379–386
Raina R, Krishnappa V (2019) An update on LDL apheresis for nephrotic syndrome. Pediatr Nephrol 34:1655–1669
Muso E, Mune M, Fujii Y, Imai E, Ueda N, Hatta K, Imada A, Takemura T, Miki S, Kuwahara T, Takamitsu Y, Tsubakihara Y; Kansai FGS LDL Apheresis Treatment (K-FLAT) Study Group (2001) Significantly rapid relief from steroid-resistant nephrotic syndrome by LDL apheresis compared with steroid monotherapy. Nephron 89:408-415
Kobayashi T, Ando Y, Umino T, Miyata Y, Muto S, Hironaka M, Asano Y, Kusano E (2006) Complete remission of minimal-change nephrotic syndrome induced by apheresis monotherapy. Clin Nephrol 65:423–426
Raina R, Krishnappa V, Sanchez-Kazi C, Quiroga A, Twombley KE, Mathias R, Lo M, Chakraborty R, Mahesh S, Steinke J, Bunchman T, Zaritsky J (2019) Dextran-sulfate plasma adsorption lipoprotein apheresis in drug resistant primary focal segmental glomerulosclerosis patients: results from a prospective, multicenter, single-arm intervention study. Front Pediatr 7:454–454
Post approval study of Liposorber LA-15 system for the treatment of focal segmental glomerulosclerosis in children. clinicaltrials.gov. NCT02235857
Author information
Authors and Affiliations
Contributions
KT designed the study. KT and MFA performed data acquisition. KT carried out data analysis. KT and MFA performed literature review. KT and MFA prepared the manuscript. All authors were involved in reviewing the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Approved by the Medical University of South Carolina Institutional Review Board.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
Dr. Twombley receives funding from Kaneka for the FDA post-approval study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Al-mousily, M., Nicoara, O., Selewski, D.T. et al. Liposorber® LA-15 system for LDL apheresis in resistant nephrotic syndrome patients. Pediatr Nephrol 37, 585–592 (2022). https://doi.org/10.1007/s00467-021-05211-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-021-05211-8