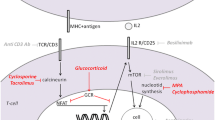
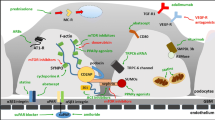
Abstract
Podocytes are the direct target of immunologic injury in many immune-mediated glomerular diseases, leading to proteinuria and subsequent kidney failure. Immunosuppressive agents such as steroids, calcineurin inhibitors, and rituximab are the commonly used treatment strategies in this context for their immunotherapeutic or anti-inflammatory properties. However, in recent years, studies have demonstrated that immunosuppressive agents can have a direct effect on podocytes, introducing the concept of the non-immunologic mechanism of kidney protection by immunomodulators. In this review, we focus on the mechanisms by which these agents may directly target the podocyte independent of their systemic effects and examine their clinical significance.

Similar content being viewed by others
References
Garg P (2018) A review of podocyte biology. Am J Nephrol 47(Suppl1):3–13. https://doi.org/10.1159/000481633
Abrahamson DR (2012) Role of the podocyte (and glomerular endothelium) in building the GBM. Semin Nephrol 32:342–349. https://doi.org/10.1016/j.semnephrol.2012.06.005
Eremina V, Jefferson JA, Kowalewska J, Hochster H, Haas M, Weisstuch J, Richardson C, Kopp JB, Kabir MG, Backx PH, Gerber HP, Ferrara N, Barisoni L, Alpers CE, Quaggin SE (2008) VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med 358:1129–1136. https://doi.org/10.1056/NEJMoa0707330
Neal CR, Crook H, Bell E, Harper SJ, Bates DO (2005) Three-dimensional reconstruction of glomeruli by electron microscopy reveals a distinct restrictive urinary subpodocyte space. J Am Soc Nephrol 16:1223–1235. https://doi.org/10.1681/ASN.2004100822
Grahammer F (2017) New structural insights into podocyte biology. Cell Tissue Res 369:5–10. https://doi.org/10.1007/s00441-017-2590-3
Yu SM, Nissaisorakarn P, Husain I, Jim B (2018) Proteinuric kidney diseases: a podocyte's slit diaphragm and cytoskeleton approach. Front Med 5:221. https://doi.org/10.3389/fmed.2018.00221
Kawachi H, Fukusumi Y (2020) New insight into podocyte slit diaphragm, a therapeutic target of proteinuria. Clin Exp Nephrol 24:193–204. https://doi.org/10.1007/s10157-020-01854-3
Kriz W, Shirato I, Nagata M, LeHir M, Lemley KV (2013) The podocyte's response to stress: the enigma of foot process effacement. Am J Physiol Ren Physiol 304:F333–F347. https://doi.org/10.1152/ajprenal.00478.2012
Kriz W, Lemley KV (2017) Potential relevance of shear stress for slit diaphragm and podocyte function. Kidney Int 91:1283–1286. https://doi.org/10.1016/j.kint.2017.02.032
Nagata M (2016) Podocyte injury and its consequences. Kidney Int 89:1221–1230. https://doi.org/10.1016/j.kint.2016.01.012
Yoo TH, Fornoni A (2015) Nonimmunologic targets of immunosuppressive agents in podocytes. Kidney Res Clin Pract 34:69–75. https://doi.org/10.1016/j.krcp.2015.03.003
Cheng X, Zhao X, Khurana S, Bruggeman LA, Kao HY (2013) Microarray analyses of glucocorticoid and vitamin D3 target genes in differentiating cultured human podocytes. PLoS One 8:e60213. https://doi.org/10.1371/journal.pone.0060213
Jiang L, Hindmarch CC, Rogers M, Campbell C, Waterfall C, Coghill J, Mathieson PW, Welsh GI (2016) RNA sequencing analysis of human podocytes reveals glucocorticoid regulated gene networks targeting non-immune pathways. Sci Rep 6:35671. https://doi.org/10.1038/srep35671
Yamauchi K, Takano Y, Kasai A, Hayakawa K, Hiramatsu N, Enomoto N, Yao J, Kitamura M (2006) Screening and identification of substances that regulate nephrin gene expression using engineered reporter podocytes. Kidney Int 70:892–900. https://doi.org/10.1038/sj.ki.5001625
Fujii Y, Khoshnoodi J, Takenaka H, Hosoyamada M, Nakajo A, Bessho F, Kudo A, Takahashi S, Arimura Y, Yamada A, Nagasawa T, Ruotsalainen V, Tryggvason K, Lee AS, Yan K (2006) The effect of dexamethasone on defective nephrin transport caused by ER stress: a potential mechanism for the therapeutic action of glucocorticoids in the acquired glomerular diseases. Kidney Int 69:1350–1359. https://doi.org/10.1038/sj.ki.5000317
Ohashi T, Uchida K, Uchida S, Sasaki S, Nitta K (2011) Dexamethasone increases the phosphorylation of nephrin in cultured podocytes. Clin Exp Nephrol 15:688–693. https://doi.org/10.1007/s10157-011-0479-0
Uchida K, Suzuki K, Iwamoto M, Kawachi H, Ohno M, Horita S, Nitta K (2008) Decreased tyrosine phosphorylation of nephrin in rat and human nephrosis. Kidney Int 73:926–932. https://doi.org/10.1038/ki.2008.19
Zha D, Chen C, Liang W, Chen X, Ma T, Yang H, Hv G, Ding G (2013) Nephrin phosphorylation regulates podocyte adhesion through the PINCH-1-ILK-α-parvin complex. BMB Rep 46:230–235. https://doi.org/10.5483/bmbrep.2013.46.4.270
Xing CY, Saleem MA, Coward RJ, Ni L, Witherden IR, Mathieson PW (2006) Direct effects of dexamethasone on human podocytes. Kidney Int 70:1038–1045. https://doi.org/10.1038/sj.ki.5001655
Yu S, Yu L (2012) Dexamethasone resisted podocyte injury via stabilizing TRPC6 expression and distribution. Evid Based Complement Alternat Med 2012:652059. https://doi.org/10.1155/2012/652059
Reiser J, von Gersdorff G, Loos M, Oh J, Asanuma K, Giardino L, Rastaldi MP, Calvaresi N, Watanabe H, Schwarz K, Faul C, Kretzler M, Davidson A, Sugimoto H, Kalluri R, Sharpe AH, Kreidberg JA, Mundel P (2004) Induction of B7-1 in podocytes is associated with nephrotic syndrome. J Clin Invest 113:1390–1397. https://doi.org/10.1172/JCI20402
Asada M, Rauch A, Shimizu H, Maruyama H, Miyaki S, Shibamori M, Kawasome H, Ishiyama H, Tuckermann J, Asahara H (2011) DNA binding-dependent glucocorticoid receptor activity promotes adipogenesis via Krüppel-like factor 15 gene expression. Lab Investig 91:203–215. https://doi.org/10.1038/labinvest.2010.170
Mallipattu SK, Guo Y, Revelo MP, Roa-Peña L, Miller T, Ling J, Shankland SJ, Bialkowska AB, Ly V, Estrada C, Jain MK, Lu Y, Ma'ayan A, Mehrotra A, Yacoub R, Nord EP, Woroniecki RP, Yang VW, He JC (2017) Krüppel-Like factor 15 mediates glucocorticoid-induced restoration of podocyte differentiation markers. J Am Soc Nephrol 28:166–184. https://doi.org/10.1681/ASN.2015060672
Han SS, Yu MY, Yoo KD, Lee JP, Kim DK, Kim YS, Yang SH (2018) Loss of KLF15 accelerates chronic podocyte injury. Int J Mol Med 42:1593–1602. https://doi.org/10.3892/ijmm.2018.3726
Mallipattu SK, Liu R, Zheng F, Narla G, Ma'ayan A, Dikman S, Jain MK, Saleem M, D'Agati V, Klotman P, Chuang PY, He JC (2012) Kruppel-like factor 15 (KLF15) is a key regulator of podocyte differentiation. J Biol Chem 287:19122–19135. https://doi.org/10.1074/jbc.M112.345983
Zhao X, Khurana S, Charkraborty S, Tian Y, Sedor JR, Bruggman LA, Kao HY (2017) α Actinin 4 (ACTN4) regulates glucocorticoid receptor-mediated transactivation and transrepression in podocytes. J Biol Chem 292:1637–1647. https://doi.org/10.1074/jbc.M116.755546
McCaffrey JC, Webb NJ, Poolman TM, Fresquet M, Moxey C, Zeef L, Donaldson IJ, Ray DW, Lennon R (2017) Glucocorticoid therapy regulates podocyte motility by inhibition of Rac1. Sci Rep 7:6725. https://doi.org/10.1038/s41598-017-06810-y
Ransom RF, Lam NG, Hallett MA, Atkinson SJ, Smoyer WE (2005) Glucocorticoids protect and enhance recovery of cultured murine podocytes via actin filament stabilization. Kidney Int 68:2473–2483. https://doi.org/10.1111/j.1523-1755.2005.00723.x
Wu J, Zheng C, Fan Y, Zeng C, Chen Z, Qin W, Zhang C, Zhang W, Wang X, Zhu X, Zhang M, Zen K, Liu Z (2014) Downregulation of microRNA-30 facilitates podocyte injury and is prevented by glucocorticoids. J Am Soc Nephrol 25:92–104. https://doi.org/10.1681/ASN.2012111101
Wada T, Pippin JW, Marshall CB, Griffin SV, Shankland SJ (2005) Dexamethasone prevents podocyte apoptosis induced by puromycin aminonucleoside: role of p53 and Bcl-2-related family proteins. J Am Soc Nephrol 16:2615–2625. https://doi.org/10.1681/ASN.2005020142
Wada T, Pippin JW, Nangaku M, Shankland SJ (2008) Dexamethasone's prosurvival benefits in podocytes require extracellular signal-regulated kinase phosphorylation. Nephron Exp Nephrol 109:e8–e19. https://doi.org/10.1159/000131892
Faul C, Donnelly M, Merscher-Gomez S, Chang YH, Franz S, Delfgaauw J, Chang JM, Choi HY, Campbell KN, Kim K, Reiser J, Mundel P (2008) The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat Med 14:931–938. https://doi.org/10.1038/nm.1857
Schlöndorff J, Del Camino D, Carrasquillo R, Lacey V, Pollak MR (2009) TRPC6 mutations associated with focal segmental glomerulosclerosis cause constitutive activation of NFAT-dependent transcription. Am J Physiol Cell Physiol 296:C558–C569. https://doi.org/10.1152/ajpcell.00077.2008
Peng L, Ma J, Cui R, Chen X, Wei SY, Wei QJ, Li B (2014) The calcineurin inhibitor tacrolimus reduces proteinuria in membranous nephropathy accompanied by a decrease in angiopoietin-like-4. PLoS One 9:e106164. https://doi.org/10.1371/journal.pone.0106164
Wang L, Chang JH, Paik SY, Tang Y, Eisner W, Spurney RF (2011) Calcineurin (CN) activation promotes apoptosis of glomerular podocytes both in vitro and in vivo. Mol Endocrinol 25:1376–1386. https://doi.org/10.1210/me.2011-0029
Li R, Zhang L, Shi W, Zhang B, Liang X, Liu S, Wang W (2013) NFAT2 mediates high glucose-induced glomerular podocyte apoptosis through increased Bax expression. Exp Cell Res 319:992–1000. https://doi.org/10.1016/j.yexcr.2013.01.007
Fornoni A, Sageshima J, Wei C, Merscher-Gomez S, Aguillon-Prada R, Jauregui AN, Li J, Mattiazzi A, Ciancio G, Chen L, Zilleruelo G, Abitbol C, Chandar J, Seeherunvong W, Ricordi C, Ikehata M, Rastaldi MP, Reiser J, Burke GW 3rd (2011) Rituximab targets podocytes in recurrent focal segmental glomerulosclerosis. Sci Transl Med 3:85ra46. https://doi.org/10.1126/scitranslmed.3002231
Yu CC, Fornoni A, Weins A, Hakroush S, Maiguel D, Sageshima J, Chen L, Ciancio G, Faridi MH, Behr D, Campbell KN, Chang JM, Chen HC, Oh J, Faul C, Arnaout MA, Fiorina P, Gupta V, Greka A, Burke GW, 3rd … Mundel P (2013) Abatacept in B7-1-positive proteinuric kidney disease. N Engl J Med 369:2416–2423. https://doi.org/10.1056/NEJMoa1304572
Nakajo A, Khoshnoodi J, Takenaka H, Hagiwara E, Watanabe T, Kawakami H, Kurayama R, Sekine Y, Bessho F, Takahashi S, Swiatecka-Urban A, Tryggvason K, Yan K (2007) Mizoribine corrects defective nephrin biogenesis by restoring intracellular energy balance. J Am Soc Nephrol 18:2554–2564. https://doi.org/10.1681/ASN.2006070732
Lee HW, Khan SQ, Faridi MH, Wei C, Tardi NJ, Altintas MM, Elshabrawy HA, Mangos S, Quick KL, Sever S, Reiser J, Gupta V (2015) A podocyte-based automated screening assay identifies protective small molecules. J Am Soc Nephrol 26:2741–2752. https://doi.org/10.1681/ASN.2014090859
Wei C, Möller CC, Altintas MM, Li J, Schwarz K, Zacchigna S, Xie L, Henger A, Schmid H, Rastaldi MP, Cowan P, Kretzler M, Parrilla R, Bendayan M, Gupta V, Nikolic B, Kalluri R, Carmeliet P, Mundel P, Reiser J (2008) Modification of kidney barrier function by the urokinase receptor. Nat Med 14:55–63. https://doi.org/10.1038/nm1696
Cheng CC, Lee YF, Lan JL, Wu MJ, Hsieh TY, Lin NN, Wang JM, Chiu YT (2013) Mycophenolate mofetil alleviates lupus nephritis through urokinase receptor signaling in a mice model. Lupus 22:554–561. https://doi.org/10.1177/0961203313480398
Gong R (2011) The renaissance of corticotropin therapy in proteinuric nephropathies. Nat Rev Nephrol 8:122–128. https://doi.org/10.1038/nrneph.2011.190
Gong R (2014) Leveraging melanocortin pathways to treat glomerular diseases. Adv Chronic Kidney Dis 21:134–151. https://doi.org/10.1053/j.ackd.2013.09.004
Ponticelli C, Locatelli F (2018) Glucocorticoids in the treatment of glomerular diseases: pitfalls and pearls. Clin J Am Soc Nephrol 13:815–822. https://doi.org/10.2215/CJN.12991117
Panettieri RA, Schaafsma D, Amrani Y, Koziol-White C, Ostrom R, Tliba O (2019) Non-genomic effects of glucocorticoids: an updated view. Trends Pharmacol Sci 40:38–49. https://doi.org/10.1016/j.tips.2018.11.002
Hosseiniyan Khatibi SM, Ardalan M, Abediazar S, Zununi Vahed S (2020) The impact of steroids on the injured podocytes in nephrotic syndrome. J Steroid Biochem Mol Biol 196:105490. https://doi.org/10.1016/j.jsbmb.2019.105490
Zhao X, Hwang DY, Kao HY (2018) The role of glucocorticoid receptors in podocytes and nephrotic syndrome. Nucl Receptor Res 5:101323. https://doi.org/10.11131/2018/101323
Martin CE, Jones N (2018) Nephrin signaling in the podocyte: an updated view of signal regulation at the slit diaphragm and beyond. Front Endocrinol 9:302. https://doi.org/10.3389/fendo.2018.00302
Schönenberger E, Ehrich JH, Haller H, Schiffer M (2011) The podocyte as a direct target of immunosuppressive agents. Nephrol Dial Transplant 26:18–24. https://doi.org/10.1093/ndt/gfq617
Brähler S, Ising C, Hagmann H, Rasmus M, Hoehne M, Kurschat C, Kisner T, Goebel H, Shankland S, Addicks K, Thaiss F, Schermer B, Pasparakis M, Benzing T, Brinkkoetter PT (2012) Intrinsic proinflammatory signaling in podocytes contributes to podocyte damage and prolonged proteinuria. Am J Physiol Ren Physiol 303:F1473–F1485. https://doi.org/10.1152/ajprenal.00031.2012
Yu M, Ren Q, Yu SY (2014) Role of nephrin phosphorylation inducted by dexamethasone and angiotensin II in podocytes. Mol Biol Rep 41:3591–3595. https://doi.org/10.1007/s11033-014-3222-6
Bohr DC, Koch M, Kritzenberger M, Fuchshofer R, Tamm ER (2011) Increased expression of olfactomedin-1 and myocilin in podocytes during puromycin aminonucleoside nephrosis. Nephrol Dial Transplant 26:83–92. https://doi.org/10.1093/ndt/gfq366
Hall G, Wang L, Spurney RF (2019) TRPC channels in proteinuric kidney diseases. Cells 9:44. https://doi.org/10.3390/cells9010044
Shimada M, Ishimoto T, Lee PY, Lanaspa MA, Rivard CJ, Roncal-Jimenez CA, Wymer DT, Yamabe H, Mathieson PW, Saleem MA, Garin EH, Johnson RJ (2012) Toll-like receptor 3 ligands induce CD80 expression in human podocytes via an NF-κB-dependent pathway. Nephrol Dial Transplant 27:81–89. https://doi.org/10.1093/ndt/gfr271
Huang Y, Border WA, Yu L, Zhang J, Lawrence DA, Noble NA (2008) A PAI-1 mutant, PAI-1R, slows progression of diabetic nephropathy. J Am Soc Nephrol 19:329–338. https://doi.org/10.1681/ASN.2007040510
Villa L, Boor P, Konieczny A, Kunter U, van Roeyen CR, Denecke B, Gan L, Neusser MA, Cohen CD, ERCB Consortium; Eitner F, Scholl T, Ostendorf T, Floege J (2013) Late angiotensin II receptor blockade in progressive rat mesangioproliferative glomerulonephritis: new insights into mechanisms. J Pathol 229:672–684. https://doi.org/10.1002/path.4151
Clement LC, Avila-Casado C, Macé C, Soria E, Bakker WW, Kersten S, Chugh SS (2011) Podocyte-secreted angiopoietin-like-4 mediates proteinuria in glucocorticoid-sensitive nephrotic syndrome. Nat Med 17:117–122. https://doi.org/10.1038/nm.2261
Anderberg RJ, Meek RL, Hudkins KL, Cooney SK, Alpers CE, Leboeuf RC, Tuttle KR (2015) Serum amyloid A and inflammation in diabetic kidney disease and podocytes. Lab Investig 95:697. https://doi.org/10.1038/labinvest.2015.38
Endlich N, Siegerist F, Endlich K (2017) Are podocytes motile? Pflugers Arch 469:951–957. https://doi.org/10.1007/s00424-017-2016-9
Yu H, Suleiman H, Kim AH, Miner JH, Dani A, Shaw AS, Akilesh S (2013) Rac1 activation in podocytes induces rapid foot process effacement and proteinuria. Mol Cell Biol 33:4755–4764. https://doi.org/10.1128/MCB.00730-13
Guo JK, Menke AL, Gubler MC, Clarke AR, Harrison D, Hammes A, Hastie ND, Schedl A (2002) WT1 is a key regulator of podocyte function: reduced expression levels cause crescentic glomerulonephritis and mesangial sclerosis. Hum Mol Genet 11:651–659. https://doi.org/10.1093/hmg/11.6.651
Palmer RE, Kotsianti A, Cadman B, Boyd T, Gerald W, Haber DA (2001) WT1 regulates the expression of the major glomerular podocyte membrane protein Podocalyxin. Curr Biol 11:1805–1809. https://doi.org/10.1016/s0960-9822(01)00560-7
Takemoto M, He L, Norlin J, Patrakka J, Xiao Z, Petrova T, Bondjers C, Asp J, Wallgard E, Sun Y, Samuelsson T, Mostad P, Lundin S, Miura N, Sado Y, Alitalo K, Quaggin SE, Tryggvason K, Betsholtz C (2006) Large-scale identification of genes implicated in kidney glomerulus development and function. EMBO J 25:1160–1174. https://doi.org/10.1038/sj.emboj.7601014
Motojima M, Kume T, Matsusaka T, Ichikawa I (2015) Foxc1 and Foxc2 cooperate in maintaining glomerular podocytes. Foxc1 and Foxc2 cooperate in maintaining glomerular podocytes. FASEB J 29:880–888. https://doi.org/10.1096/fasebj.29.1_supplement.880.8
Zhang J, Pippin JW, Krofft RD, Naito S, Liu ZH, Shankland SJ (2013) Podocyte repopulation by renal progenitor cells following glucocorticoids treatment in experimental FSGS. Am J Physiol Ren Physiol 304:F1375–F1389. https://doi.org/10.1152/ajprenal.00020.2013
Aramburu J, Heitman J, Crabtree GR (2004) Calcineurin: a central controller of signalling in eukaryotes. EMBO Rep 5:343–348. https://doi.org/10.1038/sj.embor.7400133
Plank C, Kalb V, Hinkes B, Hildebrandt F, Gefeller O, Rascher W, Arbeitsgemeinschaft für Pädiatrische Nephrologie (2008) Cyclosporin A is superior to cyclophosphamide in children with steroid-resistant nephrotic syndrome-a randomized controlled multicentre trial by the Arbeitsgemeinschaft für Pädiatrische Nephrologie. Pediatr Nephrol 23:1483–1493. https://doi.org/10.1007/s00467-008-0794-1
Cattran DC, Alexopoulos E, Heering P, Hoyer PF, Johnston A, Meyrier A, Ponticelli C, Saito T, Choukroun G, Nachman P, Praga M, Yoshikawa N (2007) Cyclosporin in idiopathic glomerular disease associated with the nephrotic syndrome : workshop recommendations. Kidney Int 72:1429–1447. https://doi.org/10.1038/sj.ki.5002553
Charbit M, Gubler MC, Dechaux M, Gagnadoux MF, Grünfeld JP, Niaudet P (2007) Cyclosporin therapy in patients with Alport syndrome. Pediatr Nephrol 22:57–63. https://doi.org/10.1007/s00467-006-0227-y
Bensman A, Niaudet P (2010) Non-immunologic mechanisms of calcineurin inhibitors explain its antiproteinuric effects in genetic glomerulopathies. Pediatr Nephrol 25:1197–1199. https://doi.org/10.1007/s00467-010-1469-2
Gellermann J, Stefanidis CJ, Mitsioni A, Querfeld U (2010) Successful treatment of steroid-resistant nephrotic syndrome associated with WT1 mutations. Pediatr Nephrol 25:1285–1289. https://doi.org/10.1007/s00467-010-1468-3
Malina M, Cinek O, Janda J, Seeman T (2009) Partial remission with cyclosporine A in a patient with nephrotic syndrome due to NPHS2 mutation. Pediatr Nephrol 24:2051–2053. https://doi.org/10.1007/s00467-009-1211-0
Rusnak F, Mertz P (2000) Calcineurin: form and function. Physiol Rev 80:1483–1521. https://doi.org/10.1152/physrev.2000.80.4.1483
Shibasaki F, Hallin U, Uchino H (2002) Calcineurin as a multifunctional regulator. J Biochem 131:1–15. https://doi.org/10.1093/oxfordjournals.jbchem.a003063
Wakamatsu A, Fukusumi Y, Hasegawa E, Tomita M, Watanabe T, Narita I, Kawachi H (2016) Role of calcineurin (CN) in kidney glomerular podocyte: CN inhibitor ameliorated proteinuria by inhibiting the redistribution of CN at the slit diaphragm. Phys Rep 4:e12679. https://doi.org/10.14814/phy2.12679
Wang Y, Jarad G, Tripathi P, Pan M, Cunningham J, Martin DR, Liapis H, Miner JH, Chen F (2010) Activation of NFAT signaling in podocytes causes glomerulosclerosis. J Am Soc Nephrol 21:1657–1666. https://doi.org/10.1681/ASN.2009121253
Cribbs JT, Strack S (2007) Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO Rep 8:939–944. https://doi.org/10.1038/sj.embor.7401062
Townsend MJ, Monroe JG, Chan AC (2010) B-cell targeted therapies in human autoimmune diseases: an updated perspective. Immunol Rev 237:264–283. https://doi.org/10.1111/j.1600-065X.2010.00945.x
Gauckler P, Shin JI, Alberici F, Audard V, Bruchfeld A, Busch M, Cheung CK, Crnogorac M, Delbarba E, Eller K, Faguer S, Galesic K, Griffin S, Hrušková Z, Jeyabalan A, Karras A, King C, Kohli HS, Maas R, Mayer G et al (2020) Rituximab in adult minimal change disease and focal segmental glomerulosclerosis—what is known and what is still unknown? Autoimmun Rev 19:102671. https://doi.org/10.1016/j.autrev.2020.102671
Bomback AS, Fervenza FC (2018) Membranous nephropathy: approaches to treatment. Am J Nephrol 47(Suppl 1):30–42. https://doi.org/10.1159/000481635
Perosa F, Favoino E, Caragnano MA, Dammacco F (2006) Generation of biologically active linear and cyclic peptides has revealed a unique fine specificity of rituximab and its possible cross-reactivity with acid sphingomyelinase-like phosphodiesterase 3b precursor. Blood 107:1070–1077. https://doi.org/10.1182/blood-2005-04-1769
Watanabe S, Hirono K, Aizawa T, Tsugawa K, Joh K, Imaizumi T, Tanaka H (2020) Podocyte sphingomyelin phosphodiesterase acid-like 3b decreases among children with idiopathic nephrotic syndrome. Clin Exp Nephrol. https://doi.org/10.1007/s10157-020-01970-0
Takahashi Y, Ikezumi Y, Saitoh A (2017) Rituximab protects podocytes and exerts anti-proteinuric effects in rat adriamycin-induced nephropathy independent of B-lymphocytes. Nephrology (Carlton) 22:49–57. https://doi.org/10.1111/nep.12737
Tasaki M, Shimizu A, Hanekamp I, Torabi R, Villani V, Yamada K (2014) Rituximab treatment prevents the early development of proteinuria following pig-to-baboon xeno-kidney transplantation. J Am Soc Nephrol 25:737–744. https://doi.org/10.1681/ASN.2013040363
Lafayette RA, Canetta PA, Rovin BH, Appel GB, Novak J, Nath KA, Sethi S, Tumlin JA, Mehta K, Hogan M, Erickson S, Julian BA, Leung N, Enders FT, Brown R, Knoppova B, Hall S, Fervenza FC (2017) A randomized controlled trial of rituximab in IgA nephropathy with proteinuria and renal dysfunction. J Am Soc Nephrol 28:1306–1313. https://doi.org/10.1681/ASN.2016060640
Hervey PS, Keam SJ (2006) Abatacept. BioDrugs 20:53–62. https://doi.org/10.2165/00063030-200620010-00004
Collins M, Ling V, Carreno BM (2005) The B7 family of immune-regulatory ligands. Genome Biol 6:223. https://doi.org/10.1186/gb-2005-6-6-223
Genovese MC, Becker JC, Schiff M, Luggen M, Sherrer Y, Kremer J, Birbara C, Box J, Natarajan K, Nuamah I, Li T, Aranda R, Hagerty DT, Dougados M (2005) Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. N Engl J Med 353:1114–1123. https://doi.org/10.1056/NEJMoa050524
Ishimoto T, Cara-Fuentes G, Wang H, Shimada M, Wasserfall CH, Winter WE, Rivard CJ, Araya CE, Saleem MA, Mathieson PW, Johnson RJ, Garin EH (2013) Serum from minimal change patients in relapse increases CD80 expression in cultured podocytes. Pediatr Nephrol 28:1803–1812. https://doi.org/10.1007/s00467-013-2498-4
Garin EH, Reiser J, Cara-Fuentes G, Wei C, Matar D, Wang H, Alachkar N, Johnson RJ (2015) Case series: CTLA4-IgG1 therapy in minimal change disease and focal segmental glomerulosclerosis. Pediatr Nephrol 30:469–477. https://doi.org/10.1007/s00467-014-2957-6
Fiorina P, Vergani A, Bassi R, Niewczas MA, Altintas MM, Pezzolesi MG, D'Addio F, Chin M, Tezza S, Ben Nasr M, Mattinzoli D, Ikehata M, Corradi D, Schumacher V, Buvall L, Yu CC, Chang JM, La Rosa S, Finzi G, Solini A, Sayegh MH (2014) Role of podocyte B7-1 in diabetic nephropathy. J Am Soc Nephrol 25:1415–1429. https://doi.org/10.1681/ASN.2013050518
Appel GB (2015) Therapy: new data do not support use of abatacept in diabetic nephropathy. Nat Rev Nephrol 11:692–694. https://doi.org/10.1038/nrneph.2015.178
Maldonado M (2018) A phase II randomized, placebo-controlled, double-blind, parallel arms, pilot study to evaluate the efficacy and safety of intravenous abatacept in treatment resistant nephrotic syndrome (focal segmental glomerulosclerosis/ minimal change disease). ClinicalTrials.gov Identifier: NCT02592798. https://www.clinicaltrials.gov/ProvidedDocs/98/NCT02592798/Prot_000.pdf. Accessed 5 Mar 2021
Hackl A, Ehren R, Weber LT (2017) Effect of mycophenolic acid in experimental, nontransplant glomerular diseases: new mechanisms beyond immune cells. Pediatr Nephrol 32:1315–1322. https://doi.org/10.1007/s00467-016-3437-y
Chang M, Chen B, Shaffner J, Dworkin LD, Gong R (2021) Melanocortin system in kidney homeostasis and disease: novel therapeutic opportunities. Front Physiol 12:651236. https://doi.org/10.3389/fphys.2021.651236
Arneil GC, Wilson HE (1953) A.C.T.H. in nephrosis. Arch Dis Child 28:372–380. https://doi.org/10.1136/adc.28.141.372
Piel CF (1956) Management of nephrosis; the use of long continued hormone therapy. Calif Med 85:152–156
Lauson HD, Forman CW, McNamara H, Mattar G, Barnett HL (1954) The effect of corticotropin (ACTH) on glomerular permeability to albumin in children with the nephrotic syndrome. J Clin Invest 33:657–664. https://doi.org/10.1172/JCI102936
Berg AL, Arnadottir M (2000) ACTH revisited—potential implications for patients with renal disease. Nephrol Dial Transplant 15:940–942. https://doi.org/10.1093/ndt/15.7.940
Ponticelli C, Passerini P, Salvadori M, Manno C, Viola BF, Pasquali S, Mandolfo S, Messa P (2006) A randomized pilot trial comparing methylprednisolone plus a cytotoxic agent versus synthetic adrenocorticotropic hormone in idiopathic membranous nephropathy. Am J Kidney Dis 47:233–240. https://doi.org/10.1053/j.ajkd.2005.10.016
Bomback AS, Canetta PA, Beck LH Jr, Ayalon R, Radhakrishnan J, Appel GB (2012) Treatment of resistant glomerular diseases with adrenocorticotropic hormone gel: a prospective trial. Am J Nephrol 36:58–67. https://doi.org/10.1159/000339287
Lindskog A, Ebefors K, Johansson ME, Stefánsson B, Granqvist A, Arnadottir M, Berg AL, Nyström J, Haraldsson B (2010) Melanocortin 1 receptor agonists reduce proteinuria. J Am Soc Nephrol 21:1290–1298. https://doi.org/10.1681/ASN.2009101025
Chhajlani V (1996) Distribution of cDNA for melanocortin receptor subtypes in human tissues. Biochem Mol Biol Int 38:73–80
Katritch V, Cherezov V, Stevens RC (2013) Structure-function of the G protein-coupled receptor superfamily. nn. Rev Pharmacol Toxicol 53:531–556. https://doi.org/10.1146/annurev-pharmtox-032112-135923
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Salvadori, M., Tsalouchos, A. How immunosuppressive drugs may directly target podocytes in glomerular diseases. Pediatr Nephrol 37, 1431–1441 (2022). https://doi.org/10.1007/s00467-021-05196-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-021-05196-4