Abstract
Background
We propose a novel clinically significant finding, de novo lupus-like glomerulonephritis (DNLLGN), in patients with autoantibodies and kidney abnormalities in pediatric liver transplant (LT) and intestinal inclusive transplants (ITx).
Methods
We describe the clinical, serologic, and histopathologic presentation and kidney outcomes in eight patients from our center found to have DNLLGN on kidney biopsy.
Results
Pediatric recipients of non-kidney solid organ transplants developed an unusual de novo immune complex glomerulonephritis with morphologic similarity to lupus nephritis. Six had isolated LT (0.9% of all pediatric LT at our center) and two had ITx (2.1% of all ITx). Five (63%) presented with nephrotic syndrome. Five patients had autoantibodies. Patients underwent kidney biopsy at a mean of 11.5 years in LT and 2.8 years in ITx after the index transplant. Biopsies demonstrated changes similar to focal or diffuse active lupus. Follow-up eGFR at a mean of 6 years after biopsy showed a mean decrease of 30 ml/min/1.73 m2 in all patients (p = 0.11).
Conclusions
DNLLGN has not been previously recognized in this clinical setting, yet 8 kidney biopsies from pediatric recipients of LT and ITx at our center in 25 years demonstrated this finding. DNLLGN appears to be an under-reported phenomenon of clinical significance.
Graphical abstract

A higher resolution version of the Graphical abstract is available as Supplementary information.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Kidney disease is a common morbidity in patients with non-kidney abdominal organ transplants. Up to 21.3% of intestinal transplant patients develop stage 5 chronic kidney disease (CKD 5) at 60 months post-transplant [1] and up to 30% of pediatric liver recipients develop chronic kidney disease (CKD) [2]. The most frequent causes of CKD and CKD 5 in liver and multivisceral solid organ transplants are attributed to calcineurin inhibitor toxicity, thrombotic microangiopathy, nephrocalcinosis, iron overload, acute tubular injury with infections, hypertension, glomerulopathy, or polyomavirus nephropathy [1,2,3,4]. De novo glomerular disease has also been associated with progressive CKD and CKD 5 in patients with liver transplant (LT), particularly IgA nephropathy and hepatitis B- and C-associated glomerulonephritis [1, 5, 6].
De novo immune complex glomerulonephritis in adults and children has been described in a limited number of post-transplant clinical situations: with hepatitis C infection [7], after kidney transplantation [8] and idiopathic without signs of systemic lupus erythematosus [9,10,11]. The pattern on biopsy has been classified in two ways when full house immunofluorescent staining is seen: as (1) lupus-like glomerulonephritis, in the case where systemic signs of lupus and lupus autoantibodies develop in the future [12], or (2) idiopathic full house nephropathy (positivity in IgG, IgM, IgA, C1q, and C3) in the case where patients never develop extra-renal signs of lupus [10]. Importantly, idiopathic full house nephropathy has correlated with poorer kidney outcomes compared to patients with similar histopathology in systemic lupus [9], but this conclusion may have been confounded by differences in immunosuppressive treatments.
Isolated autoantibody elevations after LT have been thought to be of little clinical significance, particularly in the adult liver transplant population [13]. Although some prospective studies have shown that the development of autoantibodies, particularly anti-nuclear antibody (ANA), anti-mitochondrial DNA antibody (AMA), and anti-smooth muscle antibody (ASMA) are associated with de novo autoimmune hepatitis and rejection after pediatric liver transplantation [14], this conclusion remains controversial [15], and no kidney disease was studied in these populations.
Here we report on a hitherto undescribed de novo lupus-like immune complex glomerulonephritis (DNLLGN) found on kidney biopsy in eight pediatric recipients of non-kidney abdominal organ transplants. We propose that DNLLGN may be an autoimmune disease occurring in a pediatric post-transplant setting in pediatric LT and ITx patients with signs and symptoms of kidney disease such as hematuria, proteinuria, nephrotic syndrome, malignant hypertension, or acute kidney injury (AKI).
Methods
Patients were evaluated using deidentified designations, and Institutional Review Board exemption was obtained before initiation of this study. We performed a chart review for kidney biopsies in pediatric recipients of non-kidney abdominal transplants. We included patients whose kidney biopsies demonstrated immune complex glomerulonephritis with lupus-like features. Features that qualified as “lupus like,” distinguished from other types of glomerulitis [16], included at least two of the following five pathologic features: (1) “full-house” staining, (2) ≥2+ intensity staining for C1q, (3) extra-glomerular deposits, (4) subendothelial and subepithelial deposits, and (5) endothelial tubuloreticular inclusions. Biopsies with other immune complex-mediated glomerulonephritis (e.g., IgA nephropathy) or non-glomerular pathology were excluded. A kidney pathologist (JZ) blinded to the patients’ clinical course reexamined the available light microscopy, immunofluorescence, and electron microscopy studies for the purpose of this study and scored available slides by the 2017 ISN/RPS classification of lupus nephritis. If any component of the biopsy was not available for review, the relevant data was gleaned from the review of the microscopic description in the original pathology report. Glass slides from patients 3 and 8 were not available for re-review. A single investigator (CFS) reviewed the patients’ electronic medical records for past medical history, medications, laboratory values, comorbidities, final diagnosis by their nephrologist (ongoing SLE, remission, recovery, or deceased), and kidney measures. Data were stored using REDCap software and outputs were deidentified [17]. Comorbidities including thrombosis, surgical complications, infections, episodes of rejection, and malignancy history were collected. Estimated GFRs (eGFR) were used to assess kidney function, as measured GFRs were not universally available. eGFRs were calculated based on age at time of incident biopsy. The bedside Schwartz equation was used for patients <18 years old [18] and the CKD-EPI equation [19] was used for patients ≥ 18 years old. Study end was defined as last available follow-up to May 1, 2018. The maximum eGFR for any patient was set at 89 ml/min/1.73 m2, so as not to overestimate GFR for low muscle bulk or malnutrition. All patients’ medications were standard of care by the institutional protocol for their era; e.g., prednisone was discontinued in a majority of patients 6 months after transplant.
Results
Pre-biopsy clinical characteristics
From January 1993–May 2018, 690 pediatric LT and 96 ITx were performed at UCLA Mattel Children’s Hospital. Thirteen of these patients (9 LT and 4 ITx) manifested clinical changes in urine studies and underwent kidney biopsy. Of this group, eight patients had kidney biopsies showing lupus-like features, including six with LT (66% of biopsied patients; 0.9% of all LT) and two with ITx (50% of biopsied patients; 2.1% of all ITX). The remaining five patients who had biopsies were diagnosed with drug-induced lupus, C1q nephropathy, and low grade immune complex mesangial proliferative glomerulonephritis.
The patient demographics are summarized in Table 1. All patients were less than 4 years of age at time of transplantation (mean 1.5 ± 1.3 years). The majority (63%) were Hispanic. The most common primary disease was primary biliary cirrhosis (63%). All six LT cases were complicated by portal vein or hepatic artery thrombosis in the immediate postoperative period; three required re-transplantation (patients 2, 4, 5). Four patients had a history of autoimmune disease including psoriasis (patient 7), arthritis (patient 3), and de novo autoimmune hepatitis (patients 3, 4, 6). One patient (patient 6) had prior history of post-transplant lymphoproliferative disorder (PTLD) and was in remission at time of kidney biopsy.
Patient clinical presentation and outcome after the diagnosis of clinical kidney disease can be found in Table 2. Kidney biopsies occurred at a mean time of 11.5 ± 2.5 years after LT and 2.8 years after ITx. The mean patient age at biopsy was 11.1 ± 4.3 years. Indications for kidney biopsy included nephrotic syndrome (n = 6) and persistent proteinuria/hematuria (n = 2). Two patients with nephrotic syndrome also had severe extra-renal autoimmune manifestations, including polymyositis, pericardial effusion, pleural effusion, hemolytic anemia, and leukopenia. Less severe clinical symptoms in the other patients included mild peripheral edema and hypertension. Four patients had a documented infection for which they were treated when they presented with urine abnormalities and/or changes in eGFR, which led to biopsies. The infections were bacteremia (2), urinary tract infection (1), and hemophagocytic lymphohistiocytosis (HLH), for which the triggering organism was not identified. Seven patients were on immunosuppression at the time of clinical presentation and kidney biopsy including tacrolimus (75%), cyclosporine, mycophenolate mofetil (38%), and prednisone (25%). One patient was off all immunosuppression.
Kidney biopsy findings
Seven of eight patients demonstrated an active histological lupus-like glomerulonephritis (Table 3); the eighth patient (patient 4) had significant chronicity. The majority demonstrated diffuse active features (n = 5) and the remaining had focal active features (n = 3) corresponding to 2017 ISN/RPS lupus nephritis class IV and class III, respectively. The features of activity include endocapillary hypercellularity (n = 7) with focal endocapillary neutrophils (n = 4), focal large fuchsinophilic deposits (wire loops/hyaline pseudothrombi) (n = 5), and mild interstitial inflammation (n = 6) (Figure 1A–D). Patient 7 showed severe activity with crescents and fibrinoid necrosis (Figure 1C); patient 7 had the most severe pathology at presentation. The average activity index was 4.9 ± 3.5. Mesangial hypercellularity was present in all biopsies. Some biopsies also showed mesangial sclerosis (Figure 1B).
Representative light and immunofluorescence microscopy findings on biopsy. Glomeruli from patients 6 (A–B) and 7 (C–D) demonstrating a membranoproliferative glomerulonephritis (MPGN) pattern of injury represented by mesangial and endocapillary hypercellularity and prominent capillary loop double contours (arrows panel A). Rare segmental sclerosis was present (arrow panel B) in some cases. Patient 7 showed segmental cellular crescents (arrow panel C) and large endocapillary deposits (arrow panel D). Immunofluorescence staining for IgG (E) and C1q (F) from patient 6 are shown and demonstrate bright interrupted linear glomerular capillary loop and granular mesangial staining (A: Jones silver stain; B–D: periodic acid Schiff stain). All images are 400×
Most biopsies demonstrated minimal to mild chronicity including focal global glomerulosclerosis and focal segmental glomerulosclerosis/fibrous crescents, but at most, only mild interstitial fibrosis/tubular atrophy. The average chronicity index was 2 ± 1.6. All cases showed capillary loop double contours either by light or electron microscopy. One case had focal and segmental membranous features by light microscopy. Mild to moderate arteriosclerosis and arteriolar sclerosis were present in three biopsies
Six patients had “full-house” immunofluorescence (IF) staining in at least one biopsy. All cases had deposits in mesangial, subendothelial, and subepithelial deposits by either IF (Figure 1E–F) or EM analysis (Figure 2A–B). At least two cases also showed intramembranous deposits. Rare deposits with fibrillary substructure were present in two biopsies. The biopsies with focal activity tended to have less intense IF staining compared to those cases with diffuse glomerular involvement. Three cases had tubular reticular inclusions (TRI), illustrated in Figure 2C. There was also increased glomerular extra-cellular lipid accumulation in five biopsies, which is a finding often seen in the setting of chronic liver disease. There were no extra-glomerular deposits present in any biopsy by IF or EM. There were no instances of lupus vasculopathy, vasculitis, or thrombotic microangiopathy. No biopsies showed features of calcineurin inhibitor toxicity.
Representative electron microscopy findings on biopsy. All patients exhibited varying degrees of double contour formation and subendothelial deposits. A Representative images of a glomerular capillary loops with prominent double contour formation (patient 1) represented by subendothelial neomembrane and cell process interposition associated with subendothelial deposits. B High magnification view of a double contour (patient 6) demonstrating granular electron dense immune complex deposits in subendothelial and rarely subepithelial (arrow) locations. C Glomerular capillary loops showing a cytoplasmic tubuloreticular inclusion within an endothelial cell (patient 7) (arrow)
Serologic evaluation
Serologic evaluation including C3, C4, ANA, anticardiolipin IgG and IgM, anti-beta-2-glycoprotein IgA, IgG, and IgM (B2G), anti-double-stranded DNA (dsDNA), myeloperoxidase antibody, proteinase 3-antibody, anti-liver-kidney microsome type 1 antibody (anti-LKM1), AMA, ASMA, and ribonucleoprotein antibody was available for seven patients; the missing data were collected at another institution and not available for review. Seventy-one percent of these patients had at least one positive autoantibody (Table 2) or complement marker including dsDNA (3), ANA (2), antiB2G IgA (1), anticardiolipin IgG (2), low C3 (2), and low C4 (2). Two patients had a negative serologic work up.
Post-biopsy outcomes
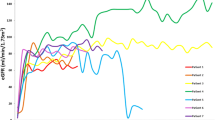
Patients were subsequently treated as a class III/IV lupus glomerulonephritis with first line of induction with steroid pulse and mycophenolate. Response to treatment was variable. The patients with focal DNLLGN all achieved clinical remission after induction without relapses, whereas the patients with diffuse glomerular disease had only partial treatment responses requiring continued therapy. Two patients had post-treatment biopsies. The morphologic features in the follow-up biopsies were similar, although the foot process effacement had improved with treatment in patient 6 but did not improve in patient 7. Four patients had ongoing CKD stage 2–4 at study end, and one expired from unexpected cardiac arrest with liver failure and CKD 5D, 68 months after kidney biopsy. Mean estimated GFR at study end was 75 mL/min/1.73 m2, corresponding to a mean decrease of 30 mL/min/1.73 m2 at the 9-year mean study end time post-biopsy; this was not a statistically significant decrease (p = 0.11) although a trend was evident as shown in Figure 3. Estimated GFR tended to be lower at follow-up in patients whose biopsies had diffuse DNLLGN, with a mean eGFR decreased from 81 to 63 mL/min/1.73 m2, noted with solid lines in Figure 3. Patients with focal DNLLGN showed stable eGFR 78–81 mL/min/1.73 m2 at study end, noted with dashed lines in Figure 3. The mean eGFR decrement was larger in ITx patients (mean decrease of 44 mL/min/1.73 m2) compared to LT (mean decrement of 33 ml/min/1.73 m2).
Discussion
We describe 8 patients in a single pediatric transplant center who were recipients of LT or ITx and who developed de novo lupus-like immune complex glomerulonephritis (DNLLGN.) This is a novel finding, and the association between glomerulonephritis with lupus characteristics, autoantibodies, and non-kidney abdominal solid organ transplantation has not been previously reported.
To our knowledge, no other studies evaluating kidney biopsies after non-kidney organ transplantation have reported cases of lupus-like glomerulonephritis [2]. Two prior cases occurring in the setting of liver transplantation have been reported as “lupus nephritis” in a single-center case series including one pediatric LT recipient and one adult LT recipient [5], diagnosed based on mesangial proliferative changes and full house immune complex deposits on IF. However, this study did not include serum autoantibodies, extra-renal clinical symptoms, complete histopathology description, or specific declines in eGFR. As such, a robust clinical association could not be hypothesized. The diagnosis of DNLLGN in our cohort was based upon the patients’ clinical trajectories as well as rigorous histologic evidence that was a characteristic of lupus nephritis, including multi-compartment immune complex deposition (i.e., co-existence of mesangial, subendothelial, subepithelial, and intramembranous deposits), large wire loop/hyaline pseudothrombi-type deposits, full house immunofluorescence staining, and the presence of tubuloreticular inclusions. Some features characteristic for lupus nephritis, namely extra-glomerular deposits, were not present in any case. Importantly, patients did not consistently have extra-renal clinical manifestations of SLE suggesting that the observed DNLLGN is predominantly a kidney-limited lupus-like glomerulonephritis, and not necessarily a manifestation of systemic collagen vascular disease. However, one patient had ongoing extra-renal disease at study end. The severity of AKI at presentation differed among patients. Taken together, our study represents the largest and most definitive case series of these findings to date and the first to report such changes in recipients of ITx.
Prior to this study, autoantibodies which have been reported to be of importance after LT are primarily ANA, ASMA, AMA, and anti-LKM1 antibody, while others think these are not significant. A prospective study showed the clinical significance of these autoantibodies in pediatric LT without history of autoimmunity, with increasing risk of chronic rejection and de novo autoimmune hepatitis [14]. Autoantibodies to endothelial antigens have also been associated with transplant complications in pediatric LT recipients [20, 21]. It is possible that the pathogenesis of autoantibodies seen in our patients with DNLLGN is similar to these other autoimmune phenomena observed following transplantation, but more studies are necessary to establish this. Interestingly, all LT with a lupus-like presentation were complicated by thrombosis of some kind; over 30 years, the percentages of operations with these complications were 7.5% for hepatic artery thrombosis and 3.2% for portal vein thrombosis [22]. But no LT had evidence of lupus anticoagulant on serology during the laboratory work up prior to transplantation.
In the setting of organ transplantation, autoimmunity could be stimulated by medications, specifically calcineurin inhibitors, vascular injury [23], or infections [24]. Interestingly, three patients presented with abnormal urine studies in the context of bacterial infections and one with HLH, of which the triggering organism was not identified. Bacterial products interact with toll-like receptors, which can provoke the production of potentially deleterious antibodies [25]. Autoimmune manifestations have also been reported with viral infections including Epstein bar virus, cytomegalovirus, and human parvovirus B19 infections [24, 25].
We found declining eGFR over a long follow-up period, in agreement with prior literature for the ITx and LT populations. Decrements in eGFR observed in these patients were likely multifactorial, due both to DNLLGN and multiple AKI episodes and drug toxicities, all of which accompany non-kidney abdominal organ transplant. It was of interest to note that patients with diffuse proliferative DNLLGN appeared to have a more pronounced decrement in eGFR than did patients with focal proliferative changes.
Our study has some important limitations including its small size and limited data collected. We were unable to obtain serologic data from one patient and pathology slides for re-review in two patients, due to the fact that the workup was performed at another institution. This study is a retrospective chart review in a single center, which inherently cannot show correlations or true prevalence. Many patients were found to have proteinuria incidentally during a work up for infection, and a prospective cohort would be better at identifying risk factors for autoantibody production and progression of CKD or glomerular disease in pediatric LT and ITx patients. We describe a decline in eGFR over time but do not have a matched cohort group to compare the decline of eGFR so we cannot say if this is the natural history after transplantation or directly related to DNLLGN. What we describe as a lupus-like presentation could be immune-mediated glomerulonephritis of post-infectious or unclear etiology or true de novo SLE in some cases. A prospective study could better differentiate between these etiologies and the role of DNLLGN in reduced kidney function. Finally, it is not clear from our data whether pediatric LT and/or ITx patients are more likely to develop DNLLGN changes than are adult patients. Furthermore, at our center, which is a large and experienced transplant center in both children and adults, DNLLGN has not been noted in adult patients, but rather only in pediatrics.
This is the most definitive report of DNLLGN in patients with LT and ITx and the largest series of its type in patients of any age. The long follow-up time in a single center is a further strength of the study. We recommend routine monitoring for proteinuria and kidney function in recipients of ITx and LT, and a nephrology referral for co-management of hypertension and abnormalities on urinalysis. In the presence of these or other signs and symptoms of changes in kidney measures, the differential diagnosis should include DNLLGN. A kidney biopsy and a full autoimmune serological workup may be necessary [5, 26]. Additional studies including multicenter surveillance of proteinuria, CKD, and glomerular diseases in LT and ITx are needed to measure the true incidence of this interesting phenomenon.
Abbreviations
- AMA:
-
anti-mitochondrial DNA antibody
- ANA:
-
anti-nuclear antibody
- anti-LKM1:
-
anti-liver-kidney microsome type 1 antibody
- ASMA:
-
anti-smooth muscle antibody
- B2G:
-
beta-2-glycoprotein
- CKD:
-
chronic kidney disease
- CKD 5:
-
stage 5 chronic kidney disease
- dAIH:
-
de novo autoimmune hepatitis
- DNLLGN:
-
de novo lupus-like glomerulonephritis
- dsDNA:
-
anti-double-stranded DNA
- eGFR:
-
estimated glomerular filtration rate
- EM:
-
electron microscopy
- GFR:
-
glomerular filtration rate
- HLH:
-
hemophagocytic lymphohistiocytosis
- IF:
-
immunofluorescence
- ITx:
-
intestinal inclusive transplant
- LT:
-
liver transplant
- MMF:
-
mycophenolic acid
- NS:
-
nephrotic syndrome
- Pd:
-
prednisone
- SLE:
-
systemic lupus erythematosus
- TRI:
-
tubular reticular inclusions
- Txp:
-
transplant
- UTI:
-
urinary tract infection
References
Ojo A, Held PJ, Friedrich PK, Wolfe RA, Leichtman AB, Young EW, Arndorfer J, Christensen L, Merion RM (2003) Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med 349:931–940
Schwarz A, Haller H, Schmitt R, Schiffer M et al (2010) Biopsy-diagnosed renal disease in patients after transplantation of other organs and tissues. Am J Transplant 10:2017–2025. https://doi.org/10.1111/j.1600-6143.2010.03224.x
Hingorani S (2008) Chronic kidney disease after liver, cardiac, lung, heart-lung, and hematopoietic stem cell transplant. Pediatr Nephrol 2:879–888. https://doi.org/10.1007/s00467-008-0785-2
Suzuki M, Mujtaba MA, Sharfuddin AA, Yaqub MS, Mishler DP, Faiz S, Vianna RM, Mangus RS, Tector JA, Taber TE (2012) Risk factors for native kidney dysfunction in patients with abdominal multivisceral/small bowel transplantation. Clin Transpl 26:E351–E358. https://doi.org/10.1111/j.1399-0012.2012.01672.x
Terzi A, Özdemir BH, Taşlıca FZ, Özdemir FN, Kırnap M, Habera M (2014) Clinicopathologic study of kidney biopsies in patients before or after liver transplant. Exp Clin Transplant 12:129–135
Sise ME, Wisocky J, Rosales IA, Chute D, Holmes JA, Corapi KM, Babitt JL, Tangren JS, Hashemi N, Lundquist AL, Williams WW, Mount DB, Andersson KL, Rennke HG, Smith RN, Colvin R, Thadhani RI, Chung RT (2016) Lupus-like immune complex mediated glomerulonephritis in patients with hepatitis C virus infection treated with oral, interferon-free, direct-acting antiviral therapy. Kidney Int Rep 1:135–143. https://doi.org/10.1016/j.ekir.2016.06.006
Ozdemir BH, Ozdemir FN, Sezer S, Colak T, Haberal M (2006) De novo glomerulonephritis in renal allografts with hepatitis C virus infection. Transplant Proc 38:492–495. https://doi.org/10.1016/j.transproceed.2005.12.109
Hariharan S, Adams MB, Brennan DC, Davis CL, First MR, Johnson CP, Ouseph R, Peddi VR, Pelz CJ, Roza AM, Vincenti F, George V (1999) Recurrent and de novo glomerular disease after renal transplantation: a report from Renal Allograft Disease Registry (RADR). Transplantation 68:635–641. https://doi.org/10.1097/00007890-199909150-00007
Rijnink EC, Teng YK, Kraaij T, Wolterbeek R, Bruijn JA, Bajema IM (2017) Idiopathic non-lupus full-house nephropathy is associated with poor renal outcome. Nephrol Dial Transplant 32:654–662. https://doi.org/10.1093/ndt/gfx020
Bajema IM, Wilhelmus S, Alpers CE, Bruijn JA et al (2018) Revision of the International Society of Nephrology/Renal Pathology Society classification for lupus nephritis: clarification of definitions, and modified National Institutes of Health activity and chronicity indices. Kidney Int 93:789–796. https://doi.org/10.1016/j.kint.2017.11.023
Huerta A, Bomback AS, Liakopoulos V, Palanisamy A, Stokes MB, D'Agati VD, Radhakrishnan J, Markowitz GS, Appel GB (2012) Renal-limited ‘lupus-like’ nephritis. Nephrol Dial Transplant 27:2337–2342. https://doi.org/10.1093/ndt/gfr663
Wen YK, Chen ML (2010) Clinicopathological study of originally non-lupus “full-house” nephropathy. Ren Fail 32:1025–1030
Pellegrini L, Parrilli G, Santonicola A, Cinquanta L, Caputo C, Ciacci C, Zingone F (2017) Lack of clinical relevance of ANA and ASMA positivity in patients with liver transplantation without a history of autoimmune diseases. Biomed Res Int:2456916. https://doi.org/10.1155/2017/2456916
Avitzur Y, Ngan BY, Lao M, Fecteau A, Ng VL (2007) Prospective evaluation of the prevalence and clinical significance of positive autoantibodies after pediatric liver transplantation. J Pediatr Gastroenterol Nutr 45:222–227. https://doi.org/10.1097/MPG.0b013e31805ce219
Chen CY, Ho MC, Wu JF, Jeng YM, Chen HL, Chang MH, Lee PH, Hu RH, Ni YH (2013) Development of autoantibodies after pediatric liver transplantation. Pediatr Transplant 17:144–148. https://doi.org/10.1111/petr.12032
Stokes MB, D'Agat VD (2019) Classification of lupus nephritis; time for a change? Adv Chronic Kidney Dis 26:323–329. https://doi.org/10.1053/j.ackd.2019.06.002
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG (2009) Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42:377–381
Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL (2009) New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20:629–637. https://doi.org/10.1681/ASN.2008030287
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150:604–612. https://doi.org/10.7326/0003-4819-150-9-200905050-00006
Wozniak LJ, Hickey MJ, Chan AP, Venick RS, Farmer DG, Busuttil RW, Reed EF, McDiarmid SV (2020) Angiotensin II type-1 receptor antibodies are associated with active allograft dysfunction following pediatric liver transplantation. Transplantation 104:2547–2556. https://doi.org/10.1097/TP.0000000000003206
Feng S, Bucuvalas JC, Demetris AJ, Burrell BE, Spain KM, Kanaparthi S, Magee JC, Ikle D, Lesniak A, Lozano JJ, Alonso EM, Bray RA, Bridges NE, Doo E, Gebel HM, Gupta NA, Himes RW, Jackson AM, Lobritto SJ, Mazariegos GV, Ng VL, Rand EB, Sherker AH, Sundaram S, Turmelle YP, Sanchez-Fueyo A (2018) Evidence of chronic allograft injury in liver biopsies from long-term pediatric recipients of liver transplants. Gastroenterology. 155:1838-1851.e7. https://doi.org/10.1053/j.gastro.2018.08.023
Venick RS, Farmer DG, Soto JR, Vargas J, Yersiz H, Kaldas FM, Agopian VG, Hiatt JR, McDiarmid SV, Busuttil RW (2018) One thousand pediatric liver transplants during thirty years: lessons learned. J Am Coll Surg 226:355–366. https://doi.org/10.1016/j.jamcollsurg.2017.12.042
Zhang Q, Reed EF (2016) The importance of non-HLA antibodies in transplantation. Nat Rev Nephrol 12:484–495
Lacaille F, Moes N, Hugot JP, Cezard JP, Goulet O, Ruemmele FM (2006) Severe dysimmune cytopenia in children treated with tacrolimus after organ transplantation. Am J Transplant 6:1072–1076. https://doi.org/10.1111/j.1600-6143.2006.01304.x
Illescas-Montes R, Corona-Castro CC, Melguizo-Rodríguez L, Ruiz C, Costela-Ruiz VJ (2019) Infectious processes and systemic lupus erythematosus. Immunology 158:153–160. https://doi.org/10.1111/imm.13103
Ruebner RL, Reese PP, Denburg MR, Abt PL, Furth SL (2013) End-stage kidney disease after pediatric nonrenal solid organ transplantation. Pediatrics 132:e1319–e1326. https://doi.org/10.1542/peds.2013-0904
Data availability statement
The data that support the findings of this study are available from the corresponding author upon request.
Author information
Authors and Affiliations
Contributions
Cristina Farkas-Skiles participated in the research design, data collection, data analysis, and the writing of the paper. Robert B Ettenger participated in the research design, data analysis, and the writing of the paper. Jonathan Zuckerman participated in the research design, data collection, data analysis, and the writing of the paper. Meghan Pearl participated in the research design and the writing of the paper. Robert Venick participated in the research design and data analysis. Patricia L Weng participated in the research design, data analysis, and the writing of the paper.
Corresponding author
Ethics declarations
Ethics approval
The project was submitted as UCLA IRB#17-001266 and was determined exempt from IRB review as not meeting the definition for human subject research.
Conflicts of interest
Dr. Jonathan E. Zuckerman is a paid consultant for Leica Biosystems; not relevant to the current study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Farkas-Skiles, C.M., Ettenger, R.B., Zuckerman, J.E. et al. De novo lupus-like glomerulonephritis after pediatric non-kidney organ transplantation. Pediatr Nephrol 37, 153–161 (2022). https://doi.org/10.1007/s00467-021-05194-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-021-05194-6