Abstract
Background
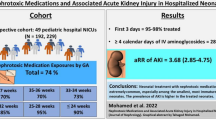
Previous studies in non-critically ill hospitalized pediatric patients have shown that daily serum creatinine monitoring for the development of nephrotoxic medication–associated acute kidney injury decreases both the rate of high nephrotoxic medication exposure and associated acute kidney injury. Attempts to spread this successful screening program have been met with concerns that daily serum creatinine monitoring in critically ill neonates with high-risk nephrotoxic medication exposure would lead to iatrogenic anemia and an increase in blood transfusion requirements.
Methods
We measured blood transfusion rates while implementing a system of daily serum creatinine monitoring in critically ill neonates at risk for high nephrotoxic medication–associated acute kidney injury.
Results
There was no correlation between blood transfusion rates and serum creatinine monitoring rates.
Conclusions
We recommend that critically ill neonates identified as having high-risk nephrotoxic medication exposure undergo daily screening for the development of nephrotoxic medication–associated acute kidney injury.

Similar content being viewed by others
Data availability
Data available
References
Askenazi DJ, Feig DI, Graham NM, Hui-Stickle S, Goldstein SL (2006) 3-5 year longitudinal follow-up of pediatric patients after acute renal failure. Kidney Int 69:184–189. https://doi.org/10.1038/sj.ki.5000032
Kaddourah A, Basu RK, Bagshaw SM, Goldstein SL (2017) Epidemiology of acute kidney injury in critically ill children and young adults. N Engl J Med 376:11–20. https://doi.org/10.1056/NEJMoa1611391
Schneider J, Khemani R, Grushkin C, Bart R (2010) Serum creatinine as stratified in the RIFLE score for acute kidney injury is associated with mortality and length of stay for children in the pediatric intensive care unit. Crit Care Med 38:933–939. https://doi.org/10.1097/CCM.0b013e3181cd12e1
Menon S, Kirkendall ES, Nguyen H, Goldstein SL (2014) Acute kidney injury associated with high nephrotoxic medication exposure leads to chronic kidney disease after 6 months. J Pediatr 165:522-527.e2. https://doi.org/10.1016/j.jpeds.2014.04.058
Goldstein SL, Kirkendall E, Nguyen H, Schaffzin JK, Bucuvalas J, Bracke T, Seid M, Ashby M, Foertmeyer N, Brunner L, Lesko A, Barclay C, Lannon C, Muething S (2013) Electronic health record identification of nephrotoxin exposure and associated acute kidney injury. Pediatrics 132:e756–e767. https://doi.org/10.1542/peds.2013-0794
Goldstein SL, Dahale D, Kirkendall ES, Mottes T, Kaplan H, Muething S, Askenazi DJ, Henderson T, Dill L, Somers MJG, Kerr J, Gilarde J, Zaritsky J, Bica V, Brophy PD, Misurac J, Hackbarth R, Steinke J, Mooney J, Ogrin S, Chadha V, Warady B, Ogden R, Hoebing W, Symons J, Yonekawa K, Menon S, Abrams L, Sutherland S, Weng P, Zhang F, Walsh K (2020) A prospective multi-center quality improvement initiative (NINJA) indicates a reduction in nephrotoxic acute kidney injury in hospitalized children. Kidney Int 97:580–588. https://doi.org/10.1016/j.kint.2019.10.015
Goldstein SL, Mottes T, Simpson K, Barclay C, Muething S, Haslam DB, Kirkendall ES (2016) A sustained quality improvement program reduces nephrotoxic medication-associated acute kidney injury. Kidney Int 90:212–221. https://doi.org/10.1016/j.kint.2016.03.031
Stoops C, Stone S, Evans E, Dill L, Henderson T, Griffin R, Goldstein SL, Coghill C, Askenazi DJ (2019) Baby NINJA (Nephrotoxic Injury Negated by Just-in-Time Action): reduction of nephrotoxic medication-associated acute kidney injury in the neonatal intensive care unit. J Pediatr 215:223-228.e6. https://doi.org/10.1016/j.jpeds.2019.08.046
Jetton JG, Boohaker LJ, Sethi SK, Wazir S, Rohatgi S, Soranno DE, Chishti AS, Woroniecki R, Mammen C, Swanson JR, Sridhar S, Wong CS, Kupferman JC, Griffin RL, Askenazi DJ (2017) Incidence and outcomes of neonatal acute kidney injury (AWAKEN): a multicentre, multinational, observational cohort study. Lancet Child Adolesc Health 1:184–194. https://doi.org/10.1016/s2352-4642(17)30069-x
Rhone ET, Carmody JB, Swanson JR, Charlton JR (2014) Nephrotoxic medication exposure in very low birth weight infants. J Matern Fetal Neonatal Med 27:1485–1490. https://doi.org/10.3109/14767058.2013.860522
Jetton JG, Guillet R, Askenazi DJ, Dill L, Jacobs J, Kent AL, Selewski DT, Abitbol CL, Kaskel FJ, Mhanna MJ, Ambalavanan N, Charlton JR (2016) Assessment of worldwide acute kidney injury epidemiology in neonates: design of a retrospective cohort study. Front Pediatr 4:68. https://doi.org/10.3389/fped.2016.00068
Askenazi DJ, Griffin R, McGwin G, Carlo W, Ambalavanan N (2009) Acute kidney injury is independently associated with mortality in very low birthweight infants: a matched case-control analysis. Pediatr Nephrol 24:991–997. https://doi.org/10.1007/s00467-009-1133-x
Benneyan JC, Lloyd RC, Plsek PE (2003) Statistical process control as a tool for research and healthcare improvement. Qual Saf Health Care 12:458–464. https://doi.org/10.1136/qhc.12.6.458
Feldman LS, Shihab HM, Thiemann D, Yeh HC, Ardolino M, Mandell S, Brotman DJ (2013) Impact of providing fee data on laboratory test ordering: a controlled clinical trial. JAMA Intern Med 173:903–908. https://doi.org/10.1001/jamainternmed.2013.232
Cassel CK, Guest JA (2012) Choosing wisely: helping physicians and patients make smart decisions about their care. JAMA 307:1801–1802. https://doi.org/10.1001/jama.2012.476
Widness JA, Madan A, Grindeanu LA, Zimmerman MB, Wong DK, Stevenson DK (2005) Reduction in red blood cell transfusions among preterm infants: results of a randomized trial with an in-line blood gas and chemistry monitor. Pediatrics 115:1299–1306. https://doi.org/10.1542/peds.2004-1680
Colombatti R, Sainati L, Trevisanuto D (2016) Anemia and transfusion in the neonate. Semin Fetal Neonatal Med 21:2–9. https://doi.org/10.1016/j.siny.2015.12.001
Author information
Authors and Affiliations
Contributions
Conceptualization: Hailey Gavigan, Cara Slagle, Brenda Poindexter, David Hooper, and Stuart Goldstein
Methodology: Hailey Gavigan, Kelli Krallman, David Hooper, and Stuart Goldstein
Formal analysis and investigation: Hailey Gavigan, Cara Slagle, Kelli Krallman, Brenda Poindexter, David Hooper, and Stuart Goldstein
Writing—original draft preparation: Hailey Gavigan
Writing—review and editing: Cara Slagle, Kelli Krallman, Brenda Poindexter, David Hooper, and Stuart Goldstein
Supervision: Brenda Poindexter, David Hooper, and Stuart Goldstein
Corresponding author
Ethics declarations
Conflicts of interest/competing interests
Cincinnati Children’s Hospital Medical Center and Dr. Stuart Goldstein have a licensing agreement with VigiLanz corporation for which they receive royalties. VigiLanz corporation markets an AKI screening algorithm to support NINJA that was used for patient identification and data analysis in this study. VigiLanz had no involvement in the design, analysis, or data interpretation of this study and did not participate in the writing or submission of the manuscript. The first draft of the manuscript was written by Hailey Gavigan who received no monetary support.
Ethics approval
IRB waiver of informed consent
Consent to participate
IRB waiver of informed consent.
Code availability
N/A
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gavigan, H.W., Slagle, C.L., Krallman, K.A. et al. Blood transfusion rates in Baby NINJA (Nephrotoxic Injury Negated by Just-in-Time Action)—a single-center experience. Pediatr Nephrol 36, 1901–1905 (2021). https://doi.org/10.1007/s00467-020-04917-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-020-04917-5