Abstract
Background
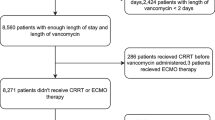
Children with kidney insufficiency are susceptible to vancomycin-induced acute kidney injury (VIAKI), but there is a lack of compelling clinical data. We conducted a nested case-control study to evaluate the relationship between kidney insufficiency and incidence of VIAKI in children.
Methods
Patients were considered to have VIAKI if they met the criteria for eGFR change according to pRIFLE-I or p-RIFLE-F. Case group comprised patients who developed VIAKI. Case-control ratio was 1:3; patients were matched for age, severity, and nature of illness and initial vancomycin dose. Primary endpoint was incidence of VIAKI at three levels of kidney function, calculated using Kaplan-Meier curve and log-rank test. Secondary endpoint was treatment-related in-hospital mortality amongst case and control groups.
Results
Amongst 386 children who fit study criteria, 31 developed VIAKI (8.03%). Thirty-one cases and 93 controls were selected from the observed cohort. Three risk factors were identified for VIAKI: moderate kidney insufficiency (OR 8.8, 2.4–32.8), vancomycin trough concentration ≥ 15 μg/mL (OR 7.7, 1.7–34.4), and furosemide use (OR 24.8, 6.4–98.2). A significant difference in time to VIAKI was noted between patients with moderate kidney insufficiency and patients with mild kidney insufficiency or normal kidney function (p < 0.001). In-hospital mortality rate in case group was 45.2%, compared to 18.3% in control group (p < 0.01).
Conclusions
Children with moderate kidney insufficiency are more likely to develop VIAKI than those with normal and mild kidney insufficiency. Patients who develop VIAKI have higher in-hospital mortality than those who do not develop VIAKI.



Similar content being viewed by others
References
Filippone EJ, Kraft WK, Farber JL (2017) The nephrotoxicity of vancomycin. Clin Pharmacol Ther 102:459–469
Jeffres MN (2017) The whole price of vancomycin: toxicities, troughs, and time. Drugs 77:1143–1154
Fiorito TM, Luther MK, Dennehy PH, LaPlante KL, Matson KL (2018) Nephrotoxicity with vancomycin in the pediatric population: a systematic review and meta-analysis. Pediatr Infect Dis J 37:654–661
Wunderink RG, Niederman MS, Kollef MH, Shorr AF, Kunkel MJ, Baruch A, McGee WT, Reisman A, Chastre J (2012) Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a randomized, controlled study. Clin Infect Dis 54:621–629
Sidi V, Roilides E, Bibashi E, Gompakis N, Tsakiri A, Koliouskas D (2000) Comparison of efficacy and safety of teicoplanin and vancomycin in children with antineoplastic therapy-associated febrile neutropenia and gram-positive bacteremia. J Chemother 12:326–331
Lodise TP, Lomaestro B, Graves J, Drusano GL (2008) Larger vancomycin doses (at least four grams per day) are associated with an increased incidence of nephrotoxicity. Antimicrob Agents Chemother 52:1330–1336
McKamy S, Hernandez E, Jahng M, Moriwaki T, Deveikis A, Le J (2011) Incidence and risk factors influencing the development of vancomycin nephrotoxicity in children. J Pediatr 158:422–4266
Sinclair EA, Yenokyan G, McMunn A, Fadrowski JJ, Milstone AM, Lee CK (2014) Factors associated with acute kidney injury in children receiving vancomycin. Ann Pharmacother 48:1555–1562
Zhang H, Wang Y, Gao P, Hu J, Chen Y, Zhang L, Shen X, Xu H, Xu Q (2016) Pharmacokinetic characteristics and clinical outcomes of vancomycin in young children with various degrees of renal function. J Clin Pharmacol 56:740–748
Lodise TP, Patel N, Lomaestro BM, Rodvold KA, Drusano GL (2009) Relationship between initial vancomycin concentration-time profile and nephrotoxicity among hospitalized patients. Clin Infect Dis 49:507–514
Karino S, Kaye KS, Navalkele B, Nishan B, Salim M, Solanki S, Pervaiz A, Tashtoush N, Shaikh H, Koppula S, Martin ET, Mynatt RP, Murray KP, Rybak MJ, Pogue JM (2016) Epidemiology of acute kidney injury among patients receiving concomitant vancomycin and piperacillin-tazobactam: opportunities for antimicrobial stewardship. Antimicrob Agents Chemother 60:3743–3750
Pollack MM, Patel KM, Ruttimann UE (1996) PRISM III: an updated pediatric risk of mortality score. Crit Care Med 24:743–752
Li Q, Cheng J, Wu Y, Wang Z, Luo S, Li Y, Tian X, Zhang G, Chen D, Luo Z (2019) Effects of delayed antibiotic therapy on outcomes in children with Streptococcus pneumoniae sepsis. Antimicrob Agents Chemother 63:e00623–e00619
Schwartz GJ, Haycock GB, Edelmann CM Jr, Spitzer A (1976) A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics 58:259–263
Macedo E, Mehta RL (2013) Measuring renal function in critically ill patients: tools and strategies for assessing glomerular filtration rate. Curr Opin Crit Care 19:560–566
Sinha Ray A, Haikal A, Hammoud KA, Yu AS (2016) Vancomycin and the risk of AKI: a systematic review and meta-analysis. Clin J Am Soc Nephrol 11:2132–2140
Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, Rybak JM, Talan DA, Chambers HF (2011) Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis 52:285–292
Akcan-Arikan A, Zappitelli M, Loftis LL, Washburn KK, Jefferson LS, Goldstein SL (2007) Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int 71:1028–1035
Navalkele B, Pogue JM, Karino S, Nishan B, Salim M, Solanki S, Pervaiz A, Tashtoush N, Shaikh H, Koppula S, Koons J, Hussain T, Perry W, Evans R, Martin ET, Mynatt RP, Murray KP, Rybak MJ, Kaye KS (2017) Risk of acute kidney injury in patients on concomitant vancomycin and piperacillin-tazobactam compared to those on vancomycin and cefepime. Clin Infect Dis 64:116–123
Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Ronco C, Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators (2005) Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 294:813–818
Bentley ML, Corwin HL, Dasta J (2010) Drug-induced acute kidney injury in the critically ill adult: recognition and prevention strategies. Crit Care Med 38:S169–S174
Totapally BR, Machado J, Lee H, Paredes A, Raszynski A (2013) Acute kidney injury during vancomycin therapy in critically ill children. Pharmacotherapy 33:598–602
Hirai T, Hanada K, Kanno A, Akashi M, Itoh T (2019) Risk factors for vancomycin nephrotoxicity and time course of renal function during vancomycin treatment. Eur J Clin Pharmacol 75:859–866
Aljefri DM, Avedissian SN, Rhodes NJ, Postelnick MJ, Nguyen K, Scheetz MH (2019) Vancomycin area under the curve and acute kidney injury: a meta-analysis. Clin Infect Dis 69:1881–1887
Tong MC, Wisniewski CS, Wolf B, Bosso JA (2016) Comparison of linezolid and vancomycin for methicillin-resistant Staphylococcus aureus pneumonia: institutional implications. Pharmacotherapy 36:731–739
Cano EL, Haque NZ, Welch VL, Cely CM, Peyrani P, Scerpella EG, Ford KD, Zervos MJ, Ramirez JA, Kett DH (2012) Incidence of nephrotoxicity and association with vancomycin use in intensive care unit patients with pneumonia: retrospective analysis of the IMPACT-HAP database. Clin Ther 34:149–157
Hall RG, Giuliano CA, Haase KK, Hazlewood KA, Frei CR, Forcade NA, Brouse SD, Bell T, Bedimo RJ, Alvarez CA (2012) Empiric guideline-recommended weight-based vancomycin dosing and mortality in methicillin-resistant Staphylococcus aureus bacteremia: a retrospective cohort study. BMC Infect Dis 12:104
Soni SS, Ronco C, Katz N, Cruz DN (2009) Early diagnosis of acute kidney injury: the promise of novel biomarkers. Blood Purif 28:165–174
Barrantes F, Tian J, Vazquez R, Amoateng-Adjepong Y, Mant-hous CA (2008) Acute kidney injury criteria predict outcomes of critically ill patients. Crit Care Med 36:1397–1403
Watkins RR, Deresinski S (2017) Increasing evidence of the nephrotoxicity of piperacillin/tazobactam and vancomycin combination therapy-what is the clinician to do? Clin Infect Dis 65:2137–2143
Funding
This work was funded by The Key Project of Science and Technology of Wuhan (2013060602010258) and National Futang Fund for Children’s Medicine Development (2015 No.33).
Author information
Authors and Affiliations
Contributions
HZ designed and organized the study; PG conducted the study and wrote the manuscript; YW analyzed the data; JC and GJ conducted the study; FZ, TF, and SY contributed to the interpretation of the data.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was approved by the ethics committee of Wuhan Children’s Hospital of Tongji Medical College of Huazhong University of Science and Technology (No. 2016070).
Consent to participate
Informed consent was obtained from all individual participants and their legal guardians.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 18 kb)
Rights and permissions
About this article
Cite this article
Zhang, H., Gao, P., Wang, Y. et al. Baseline kidney function is associated with vancomycin-induced acute kidney injury in children: a prospective nested case-control study. Pediatr Nephrol 36, 1299–1306 (2021). https://doi.org/10.1007/s00467-020-04820-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-020-04820-z