Abstract
Background
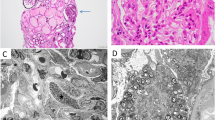
Myelin figures, or zebra bodies, seen on electron microscopy were historically considered pathognomonic of Fabry disease, a rare lysosomal storage disorder caused by alpha-galactosidase A deficiency and associated with X-linked recessive mode of inheritance. More recently, iatrogenic phospholipidosis has emerged as an important alternate cause of myelin figures in the kidney.
Methods
We report two families with autosomal dominant nephropathy presenting with proteinuria and microscopic hematuria, and the kidney biopsies were notable for the presence of myelin figures and zebra bodies.
Results
Laboratory and genetic work-up for Fabry disease was negative. Genetic testing in both families revealed the same heterozygous missense mutation in LMX1B (C.737G>A, p.Arg246Gln). LMX1B mutations are known to cause nail-patella syndrome, featuring dysplastic nails and patella with or without nephropathy, as well as isolated LMX1B-associated nephropathy in the absence of extrarenal manifestations.
Conclusions
LMX1B mutation-associated nephropathy should be considered in hereditary cases of proteinuria and/or hematuria, even in the absence of unique glomerular basement membrane changes indicative of nail-patella syndrome. In addition, LMX1B mutation should be included in the differential diagnosis of myelin figures and zebra bodies on kidney biopsy, so as to avoid a misdiagnosis.



Similar content being viewed by others
References
Hildebrandt F (2010) Genetic kidney diseases. Lancet 375:1287–1295
Zuppan CW, Weeks DA, Cutler D (2003) Nail-patella glomerulopathy without associated constitutional abnormalities. Ultrastruct Pathol 27:357–361
Isojima T, Harita Y, Furuyama M, Sugawara N, Ishizuka K, Horita S, Kajiho Y, Miura K, Igarashi T, Hattori M, Kitanaka S (2014) LMX1B mutation with residual transcriptional activity as a cause of isolated glomerulopathy. Nephrol Dial Transplant 29:81–88
Boyer O, Woerner S, Yang F, Oakeley EJ, Linghu B, Gribouval O, Tete MJ, Duca JS, Klickstein L, Damask AJ, Szustakowski JD, Heibel F, Matignon M, Baudouin V, Chantrel F, Champigneulle J, Martin L, Nitschke P, Gubler MC, Johnson KJ, Chibout SD, Antignac C (2013) LMX1B mutations cause hereditary FSGS without extrarenal involvement. J Am Soc Nephrol 24:1216–1222
Harita Y, Kitanaka S, Isojima T, Ashida A, Hattori M (2017) Spectrum of LMX1B mutations: from nail-patella syndrome to isolated nephropathy. Pediatr Nephrol 32:1845–1850
Edwards N, Rice SJ, Raman S, Hynes AM, Srivastava S, Moore I, Al-Hamed M, Xu Y, Santibanez-Koref M, Thwaites DT, Gale DP, Sayer JA (2015) A novel LMX1B mutation in a family with end-stage renal disease of ‘unknown cause’. Clin Kidney J 8:113–119
Najafian B, Smith K, Lusco MA, Alpers CE, Fogo AB (2017) AJKD atlas of renal pathology: nail-patella syndrome-associated nephropathy. Am J Kidney Dis 70:e19–e20
Nakao S, Kodama C, Takenaka T, Tanaka A, Yasumoto Y, Yoshida A, Kanzaki T, Enriquez AL, Eng CM, Tanaka H, Tei C, Desnick RJ (2003) Fabry disease: detection of undiagnosed hemodialysis patients and identification of a “renal variant” phenotype. Kidney Int 64:801–807
Ko YH, Kim HJ, Roh YS, Park CK, Kwon CK, Park MH (1996) Atypical Fabry’s disease. An oligosymptomatic variant. Arch Pathol Lab Med 120:86–89
Najafian B, Fogo AB, Lusco MA, Alpers CE (2015) AJKD atlas of renal pathology: Fabry nephropathy. Am J Kidney Dis 66:e35–e36
Grafft CA, Fervenza FC, Semret MH, Orloff S, Sethi S (2009) Renal involvement in Neimann-Pick disease. NDT Plus 2:448–451
Colpart P, Felix S (2017) Fabry nephropathy. Arch Pathol Lab Med 141:1127–1131
Banks DE, Milutinovic J, Desnick RJ, Grabowski GA, Lapp NL, Boehlecke BA (1983) Silicon nephropathy mimicking Fabry’s disease. Am J Nephrol 3:279–284
Scheurle C, Dammrich M, Becker JU, Baumgartel MW (2014) Renal phospholipidosis possibly induced by ranolazine. Clin Kidney J 7:62–64
Costa RM, Martul EV, Reboredo JM, Cigarran S (2013) Curvilinear bodies in hydroxychloroquine-induced renal phospholipidosis resembling Fabry disease. Clin Kidney J 6:533–536
Albay D, Adler SG, Philipose J, Calescibetta CC, Romansky SG, Cohen AH (2005) Chloroquine-induced lipidosis mimicking Fabry disease. Mod Pathol 18:733–738
Breiden B, Sandhoff K (2019) Emerging mechanisms of drug-induced phospholipidosis. Biol Chem 401:31–46
Silverman ME, Goodman RM, Cuppage FE (1967) The nail-patella syndrome. Clinical findings and ultrastructural observations in the kidney. Arch Intern Med 120:68–74
Andeen NK, Schleit J, Blosser CD, Dorschner MO, Hisama FM, Smith KD (2018) LMX1B-associated nephropathy with type III collagen deposition in the glomerular and tubular basement membranes. Am J Kidney Dis 72:296–301
Ghoumid J, Petit F, Holder-Espinasse M, Jourdain AS, Guerra J, Dieux-Coeslier A, Figeac M, Porchet N, Manouvrier-Hanu S, Escande F (2016) Nail-patella syndrome: clinical and molecular data in 55 families raising the hypothesis of a genetic heterogeneity. Eur J Hum Genet 24:44–50
Jones MC, Topol SE, Rueda M, Oliveira G, Phillips T, Spencer EG, Torkamani A (2017) Mutation of WIF1: a potential novel cause of a nail-patella-like disorder. Genet Med 19:1179–1183
Salcedo JR (1984) An autosomal recessive disorder with glomerular basement membrane abnormalities similar to those seen in the nail patella syndrome: report of a kindred. Am J Med Genet 19:579–584
Bongers EM, Huysmans FT, Levtchenko E, de Rooy JW, Blickman JG, Admiraal RJ, Huygen PL, Cruysberg JR, Toolens PA, Prins JB, Krabbe PF, Borm GF, Schoots J, van Bokhoven H, van Remortele AM, Hoefsloot LH, van Kampen A, Knoers NV (2005) Genotype-phenotype studies in nail-patella syndrome show that LMX1B mutation location is involved in the risk of developing nephropathy. Eur J Hum Genet 13:935–946
Lemley KV (2009) Kidney disease in nail-patella syndrome. Pediatr Nephrol 24:2345–2354
Nakata T, Ishida R, Mihara Y, Fujii A, Inoue Y, Kusaba T, Isojima T, Harita Y, Kanda C, Kitanaka S, Tamagaki K (2017) Steroid-resistant nephrotic syndrome as the initial presentation of nail-patella syndrome: a case of a de novo LMX1B mutation. BMC Nephrol 18:100
Negrisolo S, Carraro A, Fregonese G, Benetti E, Schaefer F, Alberti M, Melchionda S, Fischetto R, Giordano M, Murer L (2018) Could the interaction between LMX1B and PAX2 influence the severity of renal symptoms? Eur J Hum Genet 26:1708–1712
Gee HY, Zhang F, Ashraf S, Kohl S, Sadowski CE, Vega-Warner V, Zhou W, Lovric S, Fang H, Nettleton M, Zhu JY, Hoefele J, Weber LT, Podracka L, Boor A, Fehrenbach H, Innis JW, Washburn J, Levy S, Lifton RP, Otto EA, Han Z, Hildebrandt F (2015) KANK deficiency leads to podocyte dysfunction and nephrotic syndrome. J Clin Invest 125:2375–2384
Bedin M, Boyer O, Servais A, Li Y, Villoing-Gaude L, Tete MJ, Cambier A, Hogan J, Baudouin V, Krid S, Bensman A, Lammens F, Louillet F, Ranchin B, Vigneau C, Bouteau I, Isnard-Bagnis C, Mache CJ, Schafer T, Pape L, Godel M, Huber TB, Benz M, Klaus G, Hansen M, Latta K, Gribouval O, Moriniere V, Tournant C, Grohmann M, Kuhn E, Wagner T, Bole-Feysot C, Jabot-Hanin F, Nitschke P, Ahluwalia TS, Kottgen A, Andersen CBF, Bergmann C, Antignac C, Simons M (2019) Human C-terminal CUBN variants associate with chronic proteinuria and normal renal function. J Clin Invest 130:335–344
Kakita T, Shimohata H, Fujita S, Ogawa Y, Nagai M, Hirayama K, Nakamura H, Kobayashi M (2012) A case of focal segmental glomerulosclerosis with myeloid bodies. Ren Fail 34:801–803
Ren H, Li L, Yu J, Wu S, Zhou S, Zheng Y, Sun W (2019) Fabry disease and immunoglobulin A nephropathy presenting with Alport syndrome-like findings: a case report. Medicine (Baltimore) 98:e16256
Suleiman H, Heudobler D, Raschta AS, Zhao Y, Zhao Q, Hertting I, Vitzthum H, Moeller MJ, Holzman LB, Rachel R, Johnson R, Westphal H, Rascle A, Witzgall R (2007) The podocyte-specific inactivation of Lmx1b, Ldb1 and E2a yields new insight into a transcriptional network in podocytes. Dev Biol 304:701–712
Heidet L, Bongers EM, Sich M, Zhang SY, Loirat C, Meyrier A, Broyer M, Landthaler G, Faller B, Sado Y, Knoers NV, Gubler MC (2003) In vivo expression of putative LMX1B targets in nail-patella syndrome kidneys. Am J Pathol 163:145–155
Burghardt T, Kastner J, Suleiman H, Rivera-Milla E, Stepanova N, Lottaz C, Kubitza M, Boger CA, Schmidt S, Gorski M, de Vries U, Schmidt H, Hertting I, Kopp J, Rascle A, Moser M, Heid IM, Warth R, Spang R, Wegener J, Mierke CT, Englert C, Witzgall R (2013) LMX1B is essential for the maintenance of differentiated podocytes in adult kidneys. J Am Soc Nephrol 24:1830–1848
Laguna A, Schintu N, Nobre A, Alvarsson A, Volakakis N, Jacobsen JK, Gomez-Galan M, Sopova E, Joodmardi E, Yoshitake T, Deng Q, Kehr J, Ericson J, Svenningsson P, Shupliakov O, Perlmann T (2015) Dopaminergic control of autophagic-lysosomal function implicates Lmx1b in Parkinson’s disease. Nat Neurosci 18:826–835
Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y (2004) In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell 15:1101–1111
Hartleben B, Godel M, Meyer-Schwesinger C, Liu S, Ulrich T, Kobler S, Wiech T, Grahammer F, Arnold SJ, Lindenmeyer MT, Cohen CD, Pavenstadt H, Kerjaschki D, Mizushima N, Shaw AS, Walz G, Huber TB (2010) Autophagy influences glomerular disease susceptibility and maintains podocyte homeostasis in aging mice. J Clin Invest 120:1084–1096
Seranova E, Connolly KJ, Zatyka M, Rosenstock TR, Barrett T, Tuxworth RI, Sarkar S (2017) Dysregulation of autophagy as a common mechanism in lysosomal storage diseases. Essays Biochem 61:733–749
Konomoto T, Imamura H, Orita M, Tanaka E, Moritake H, Sato Y, Fujimoto S, Harita Y, Hisano S, Yoshiura K, Nunoi H (2016) Clinical and histological findings of autosomal dominant renal-limited disease with LMX1B mutation. Nephrology (Carlton) 21:765–773
Acknowledgements
The authors would like to thank Ms. Ivy L. Liu for her help with Figure 1.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was approved by the Institutional Review Board at Stanford University.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lei, L., Oh, G., Sutherland, S. et al. Myelin bodies in LMX1B-associated nephropathy: potential for misdiagnosis. Pediatr Nephrol 35, 1647–1657 (2020). https://doi.org/10.1007/s00467-020-04564-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-020-04564-w