Abstract
Background
Prenatal ethanol exposure has been shown to reduce nephron endowment in animal models, but the effect of alcohol during human pregnancy on postnatal kidney function has not been explored. We aim to investigate the potential association of maternal alcohol consumption during pregnancy with the offspring renal function, considering potential confounding by intrauterine growth and children’s current nutritional status.
Methods
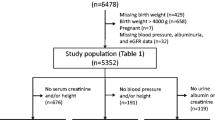
Prospective longitudinal study in a random sample of 1093 children from a population-based birth cohort. Anthropometrics and estimated glomerular filtration rate (eGFR) were assessed at 7 years of age. Multiple linear regression models were fitted, adjusting for child’s gender, age, birthweight, and maternal age, education, prepregnancy nutritional status, and smoking.
Results
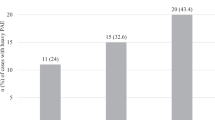
Thirteen percent of mothers consumed alcohol during pregnancy. At 7 years of age, eGFR was significantly lower in children with prenatal alcohol exposure (134 ± 17 vs.138 ± 16 mL/min/1.73m2, p = 0.014). The effect was dose dependent and only present in overweight and obese children, among whom adjusted eGFR was −6.6(−12.0 to −1.1)mL/min/1.73m2 and −11.1(−21.3 to −1.2)mL/min/1.73m2 in those exposed to ≤ 40 g and to > 40 g of alcohol per week, respectively, compared to no consumption (ptrend = 0.002).
Conclusions
Prenatal alcohol exposure has a dose-dependent adverse effect on renal function at school age in overweight and obese children.

Similar content being viewed by others
References
Ritz E, Amann K, Koleganova N, Benz K (2011) Prenatal programming-effects on blood pressure and renal function. Nat Rev Nephrol 7:137–144
Kuure S, Vuolteenaho R, Vainio S (2000) Kidney morphogenesis: cellular and molecular regulation. Mech Dev 92:31–45
Kingdom JC, Hayes M, McQueen J et al (1999) Intrauterine growth restriction is associated with persistent juxtamedullary expression of renin in the fetal kidney. Kidney Int 55:424–429
Moreau E, Vilar J, Lelièvre-Pégorier M et al (1998) Regulation of c-ret expression by retinoic acid in rat metanephros: implication in nephron mass control. Am J Phys 275:F938–F945
Woods LL, Ingelfinger JR, Nyengaard JR, Rasch R (2001) Maternal protein restriction suppresses the newborn renin-angiotensin system and programs adult hypertension in rats. Pediatr Res 49:460–467
Dagan A, Gattineni J, Cook V, Baum M (2007) Prenatal programming of rat proximal tubule Na+/H+ exchanger by dexamethasone. Am J Phys Regul Integr Comp Phys 292:R1230–R1235
Vikse BE, Irgens LM, Leivestad T et al (2008) Low birth weight increases risk for end-stage renal disease. J Am Soc Nephrol 19:151–157
Lackland DT, Egan BM, Fan ZJ, Syddall HE (2001) Low birth weight contributes to the excess prevalence of end-stage renal disease in African Americans. J Clin Hypertens 3:29–31
White SL, Perkovic V, Cass A et al (2009) Is low birth weight an antecedent of CKD in later life? a systematic review of observational studies. Am J Kidney Dis 54:248–261
Hallan S, Euser AM, Irgens LM et al (2008) Effect of intrauterine growth restriction on kidney function at young adult age: the Nord Trøndelag Health (HUNT 2) Study. Am J Kidney Dis 51:10–20
Vehaskari VM (2010) Prenatal programming of kidney disease. Curr Opin Pediatr 22:176–182
Baum M (2010) Role of the kidney in the prenatal and early postnatal programming of hypertension. Am J Physiol Ren Physiol 298:F235–F247
Moritz KM, Mazzuca MQ, Siebel AL et al (2009) Uteroplacental insufficiency causes a nephron deficit, modest renal insufficiency but no hypertension with ageing in female rats. J Physiol 587:2635–2646
Brenner BM, Garcia DL, Anderson S (1988) Glomeruli and blood pressure. Less of one, more the other? Am J Hypertens 1:335–347
Parkington HC, Coleman HA, Wintour EM, Tare M (2010) Prenatal alcohol exposure: implications for cardiovascular function in the fetus and beyond. Clin Exp Pharmacol Physiol 37:e91–e98
Parkington HC, Kenna KR, Sozo F et al (2014) Maternal alcohol consumption in pregnancy enhances arterial stiffness and alters vasodilator function that varies between vascular beds in fetal sheep. J Physiol 592:2591–2603
Gray SP, Denton KM, Cullen-McEwen L et al (2010) Prenatal exposure to alcohol reduces nephron number and raises blood pressure in progeny. J Am Soc Nephrol 21:1891–1902
Moore CA, Khoury MJ, Liu Y (1997) Does light-to-moderate alcohol consumption during pregnancy increase the risk for renal anomalies among offspring? Pediatrics 99:E11
Epstein FH, Brenner BM, Meyer TW, Hostetter TH (1982) Dietary protein intake and the progressive nature of kidney disease: the role of hemodynamically mediated glomerular injury in the pathogenesis of progressive glomerular sclerosis in aging, renal ablation, and intrinsic renal disease. N Engl J Med 307:652–659
Abitbol CL, Chandar J, Rodríguez MM et al (2009) Obesity and preterm birth: additive risks in the progression of kidney disease in children. Pediatr Nephrol 24:1363–1370
Cignarelli M, Lamacchia O (2007) Obesity and kidney disease. Nutr Metab Cardiovasc Dis 17:757–762
Gunta SS, Mak RH (2013) Is obesity a risk factor for chronic kidney disease in children? Pediatr Nephrol 28:1949–1956
Nenov VD, Taal MW, Sakharova OV, Brenner BM (2000) Multi-hit nature of chronic renal disease. Curr Opin Nephrol Hypertens 9:85–97
Silverwood RJ, Pierce M, Hardy R et al (2013) Low birth weight, later renal function, and the roles of adulthood blood pressure, diabetes, and obesity in a British birth cohort. Kidney Blood Press Res 84:1262–1270
Silverwood RJ, Pierce M, Hardy R et al (2013) Early-life overweight trajectory and CKD in the 1946 British birth cohort study. Am J Kidney Dis 62:276–284
Larsen PS, Kamper-Jorgensen M, Adamson A et al (2013) Pregnancy and birth cohort resources in Europe: a large opportunity for aetiological child health research. Paediatr Perinat Epidemiol 27:393–414
(1998) Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: executive summary. Expert Panel on the Identification, Evaluation, and Treatment of Overweight in Adults. Am J Clin Nutr 68:899–917.
Rasmussen K, Yaktine A (2009) Weight gain during pregnancy: reexamining the guidelines; Washington (DC): National Academies Press (US) 2009
Fenton TR, Kim JH (2013) A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr 13:59
Hoyme HE, Kalberg WO, Elliott AJ et al (2016) Updated clinical guidelines for diagnosing fetal alcohol spectrum disorders. Pediatrics 138:e20154256
Durão C, Severo M, Oliveira A et al (2014) Evaluating the effect of energy-dense foods consumption on preschool children’s body mass index: a prospective analysis from 2 to 4 years of age. Eur J Nutr 54:835–843
de Onis M, Onyango AW, Borghi E et al (2007) Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ 85:660–667
Flynn JT, Kaelber DC, Baker-Smith CM et al (2017) Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics 140:e20171904
Zappitelli M, Parvex P, Joseph L et al (2006) Derivation and validation of cystatin C-based prediction equations for GFR in children. Am J Kidney Dis 48:221–230
Hutchinson D, Moore EA, Breen C et al (2013) Alcohol use in pregnancy: prevalence and predictors in the Longitudinal Study of Australian Children. Drug Alcohol Rev 32:475–482
Mills JL, Graubard BI, Harley EE et al (1984) Maternal alcohol consumption and birth weight. How much drinking during pregnancy is safe? JAMA 252:1875–1879
Dörrie N, Föcker M, Freunscht I, Hebebrand J (2014) Fetal alcohol spectrum disorders. Eur Child Adolesc Psychiatry 23:863–875
Taylor CL, Jones KL, Jones MC, Kaplan GW (1994) Incidence of renal anomalies in children prenatally exposed to ethanol. Pediatrics 94:209–212
Qazi Q, Masakawa A, Milman D et al (1979) Renal anomalies in fetal alcohol syndrome. Pediatrics 63:886–889
Havers W, Majewski F, Olbing H, Eickenberg HU (1980) Anomalies of the kidneys and genitourinary tract in alcoholic embryopathy. J Urol 124:108–110
Gray SP, Kenna K, Bertram JF et al (2008) Repeated ethanol exposure during late gestation decreases nephron endowment in fetal sheep. Am J Phys Regul Integr Comp Phys 295:R568–R574
O’Leary CM, Taylor C, Zubrick SR et al (2013) Prenatal alcohol exposure and educational achievement in children aged 8-9 years. Pediatrics 132:e468–e475
Alati R, Davey Smith G, Lewis SJ et al (2013) Effect of prenatal alcohol exposure on childhood academic outcomes: contrasting maternal and paternal associations in the ALSPAC study. PLoS One 8:e74844
(2008) National Collaborating Centre for Women’s and Children’s Health. Antenatal Care: Routine Care for the Healthy Pregnant Woman. NICE Clin Guidel RCOG Press 62:1–58.
Gilliam DM, Mantle MA, Barkhausen DA, Tweden DR (1997) Effects of acute prenatal ethanol administration in a reciprocal cross of C57BL/6 J and short-sleep mice: maternal effects and nonmaternal factors. Alcohol Clin Exp Res 21:28–34
Assadi FK, Zajac CS (1992) Ultrastructural changes in the rat kidney following fetal exposure to ethanol. Alcohol 9:509–512
Assadi FK, Manaligod JR, Fleischmann LE, Zajac CS (1991) Effects of prenatal ethanol exposure on postnatal renal function and structure in the rat. Alcohol 8:259–263
Assadi FK (1990) Renal tubular dysfunction in fetal alcohol syndrome. Pediatr Nephrol 4:48–51
Mathew AV, Okada S, Sharma K (2011) Obesity related kidney disease. Curr Diabetes Rev 7:41–49
Bacchetta J, Cochat P, Rognant N et al (2011) Which creatinine and cystatin C equations can be reliably used in children? Clin J Am Soc Nephrol 6:552–560
Andersen TB, Eskild-Jensen A, Frøkiaer J, Brøchner-Mortensen J (2009) Measuring glomerular filtration rate in children; can cystatin C replace established methods? A review. Pediatr Nephrol 24:929–941
Baña A, Tabernero MJ, Pérez-Muñuzuri A et al (2014) Prenatal alcohol exposure and its repercussion on newborns. J Neonatal-Perinatal Med 7:47–54
Acknowledgments
The authors gratefully acknowledge the families enrolled in Generation XXI for their kindness, all members of the research team for their enthusiasm and perseverance, and the participating hospitals and their staff for their help and support.
Liane Correia-Costa and Franz Schaefer contributed equally to this work.
Funding
This work was supported by FEDER funds from Programa Operacional Factores de Competitividade—COMPETE [grant number FCOMP-01-0124-FEDER-028751—and national funds from the Portuguese Foundation for Science and Technology, Lisbon, Portugal [grant number PTDC/DTP-PIC/0239/2012, SFRH/SINTD/95898/2013 to Liane Correia-Costa]. Generation XXI was funded by Programa Operacional de Saúde—Saúde XXI, Quadro Comunitário de Apoio III—by Administração Regional de Saúde Norte (Regional Department of Ministry of Health) [grant number 15-01-01-FDR-00197] and by Foundation Calouste Gulbenkian. Franz Schaefer was supported by the ERA-EDTA Research Programme and the KfH Foundation for Preventive Medicine.
Author information
Authors and Affiliations
Contributions
Liane Correia-Costa and Franz Schaefer conceptualized and designed the study, analyzed the data and drafted the initial manuscript, and approved the final manuscript as submitted. Alberto Caldas Afonso, Sofia Correia, and António Guerra conceptualized and designed the study, participated in the initial analyses, reviewed and revised the manuscript, and approved the final manuscript as submitted. João Tiago Guimarães was responsible for all laboratory measurements and critically reviewed the manuscript and approved the final manuscript as submitted. Henrique Barros and Ana Azevedo conceptualized and designed the study, supervised data collection, participated in and supervised data analyses, reviewed and revised the manuscript, and approved the final manuscript as submitted. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Correia-Costa, L., Schaefer, F., Afonso, A.C. et al. Prenatal alcohol exposure affects renal function in overweight schoolchildren: birth cohort analysis. Pediatr Nephrol 35, 695–702 (2020). https://doi.org/10.1007/s00467-019-04429-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-019-04429-x