Abstract
Membranous nephropathy (MN) is an immune complex-mediated cause of the nephrotic syndrome that can occur in all age groups, from infants to the very elderly. However, nephrotic syndrome in children is more frequently caused by conditions such as minimal change disease or focal segmental glomerulosclerosis, and much less commonly by MN. While systemic conditions such as lupus or infections such as hepatitis B may more commonly be associated as secondary causes with MN in the younger population, primary or “idiopathic” MN has generally been considered a disease of adults. Autoantibodies both to the M-type phospholipase A2 receptor (PLA2R) and to thrombospondin type-1 domain-containing 7A (THSD7A), initially described in adult MN, have now been identified in children and adolescents with MN and serve as a useful diagnostic and monitoring tool in this younger population as well. Whereas definitive therapy for secondary forms of MN should be targeted at the underlying cause, immunosuppressive therapy is often necessary for primary disease. Rituximab has been successfully used in the treatment of MN, and is likely effective in children with MN as well, although dosing in the pediatric population is not well established. This review highlights the new findings in adult and pediatric MN since last reviewed in this journal.


Similar content being viewed by others
References
Ayalon R, Beck LH Jr (2015) Membranous nephropathy: not just a disease for adults. Pediatr Nephrol 30:31–39
Menon S, Valentini RP (2010) Membranous nephropathy in children: clinical presentation and therapeutic approach. Pediatr Nephrol 25:1419–1428
Larsen CP, Cossey LN, Beck LH (2016) THSD7A staining of membranous glomerulopathy in clinical practice reveals cases with dual autoantibody positivity. Mod Pathol 29:421–426
Tian C, Li L, Liu T, Qu X, Qiu Y (2019) Circulating antibodies against M-type phospholipase A2 receptor and thrombospondin type-1 domain-containing 7A in Chinese patients with membranous nephropathy. Int Urol Nephrol 51:1371–1377
Zaghrini C, Seitz-Polski B, Justino J, Dolla G, Payre C, Jourde-Chiche N, Van de Logt AE, Booth C, Rigby E, Lonnbro-Widgren J, Nystrom J, Mariat C, Cui Z, Wetzels JFM, Ghiggeri G, Beck LH Jr, Ronco P, Debiec H, Lambeau G (2019) Novel ELISA for thrombospondin type 1 domain-containing 7A autoantibodies in membranous nephropathy. Kidney Int 95:666–679
Sethi S, Madden BJ, Debiec H, Charlesworth MC, Gross L, Ravindran A, Hummel AM, Specks U, Fervenza FC, Ronco P (2019) Exostosin 1/exostosin 2-associated membranous nephropathy. J Am Soc Nephrol 30:1123–1136
Moxey-Mims MM, Stapleton FB, Feld LG (1994) Applying decision analysis to management of adolescent idiopathic nephrotic syndrome. Pediatr Nephrol 8:660–664
Mubarak M, Kazi JI, Lanewala A, Hashmi S, Akhter F (2012) Pathology of idiopathic nephrotic syndrome in children: are the adolescents different from young children? Nephrol Dial Transplant 27:722–726
Nie S, He W, Huang T, Liu D, Wang G, Geng J, Chen N, Xu G, Zhang P, Luo Y, Nie J, Xu X, Hou FF (2018) The spectrum of biopsy-proven glomerular diseases among children in China: a national, cross-sectional survey. Clin J Am Soc Nephrol 13:1047–1054
Zhang XD, Cui Z, Zhao MH (2018) The genetic and environmental factors of primary membranous nephropathy: an overview from China. Kidney Dis (Basel) 4:65–73
Zhu P, Zhou FD, Wang SX, Zhao MH, Wang HY (2015) Increasing frequency of idiopathic membranous nephropathy in primary glomerular disease: a 10-year renal biopsy study from a single Chinese nephrology centre. Nephrology (Carlton) 20:560–566
Xu X, Wang G, Chen N, Lu T, Nie S, Xu G, Zhang P, Luo Y, Wang Y, Wang X, Schwartz J, Geng J, Hou FF (2016) Long-term exposure to air pollution and increased risk of membranous nephropathy in China. J Am Soc Nephrol 27:3739–3746
Fidan K, Isik Gonul I, Buyukkaragoz B, Isiyel E, Arinsoy T, Soylemezoglu O (2016) Changing trends in pediatric renal biopsies: analysis of pediatric renal biopsies in national nephrology registry data. Ren Fail 38:1228–1233
Liu J, Liang W, Jing W, Liu M (2019) Countdown to 2030: eliminating hepatitis B disease, China. Bull World Health Organ 97:230–238
Couser WG (2017) Primary membranous nephropathy. Clin J Am Soc Nephrol 12:983–997
Chen A, Frank R, Vento S, Crosby V, Chandra M, Gauthier B, Valderrama E, Trachtman H (2007) Idiopathic membranous nephropathy in pediatric patients: presentation, response to therapy, and long-term outcome. BMC Nephrol 8:11
Liu A, Wu H, Su Y, Wang L, Xu G (2015) Idiopathic membranous nephropathy in children in China. Fetal Pediatr Pathol 34:185–189
Ponticelli C, Glassock RJ (2014) Glomerular diseases: membranous nephropathy—a modern view. Clin J Am Soc Nephrol 9:609–616
National Institutes of Health NIoDaDaKD, U.S. Renal Data System (2012) USRDS 2012 annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States, Bethesda, MD
Farquhar MG, Saito A, Kerjaschki D, Orlando RA (1995) The Heymann nephritis antigenic complex: megalin (gp330) and RAP. J Am Soc Nephrol 6:35–47
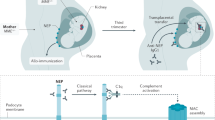
Debiec H, Guigonis V, Mougenot B, Decobert F, Haymann JP, Bensman A, Deschenes G, Ronco PM (2002) Antenatal membranous glomerulonephritis due to anti-neutral endopeptidase antibodies. N Engl J Med 346:2053–2060
Beck LH Jr, Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, Klein JB, Salant DJ (2009) M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 361:11–21
Borza DB (2016) Alternative pathway dysregulation and the conundrum of complement activation by IgG4 immune complexes in membranous nephropathy. Front Immunol 7:157
Ronco P, Debiec H (2015) Pathophysiological advances in membranous nephropathy: time for a shift in patient's care. Lancet 385:1983–1992
Liu Y, Li X, Ma C, Wang P, Liu J, Su H, Zhuo H, Kong X, Xu D, Xu D (2018) Serum anti-PLA2R antibody as a diagnostic biomarker of idiopathic membranous nephropathy: the optimal cut-off value for Chinese patients. Clin Chim Acta 476:9–14
Timmermans SA, Damoiseaux JG, Heerings-Rewinkel PT, Ayalon R, Beck LH Jr, Schlumberger W, Salant DJ, van Paassen P, Tervaert JW, Registry LR (2014) Evaluation of anti-PLA2R1 as measured by a novel ELISA in patients with idiopathic membranous nephropathy: a cohort study. Am J Clin Pathol 142:29–34
van de Logt AE, Hofstra JM, Wetzels JF (2015) Serum anti-PLA2R antibodies can be initially absent in idiopathic membranous nephropathy: seroconversion after prolonged follow-up. Kidney Int 87:1263–1264
Stanescu HC, Arcos-Burgos M, Medlar A, Bockenhauer D, Kottgen A, Dragomirescu L, Voinescu C, Patel N, Pearce K, Hubank M, Stephens HA, Laundy V, Padmanabhan S, Zawadzka A, Hofstra JM, Coenen MJ, den Heijer M, Kiemeney LA, Bacq-Daian D, Stengel B, Powis SH, Brenchley P, Feehally J, Rees AJ, Debiec H, Wetzels JF, Ronco P, Mathieson PW, Kleta R (2011) Risk HLA-DQA1 and PLA(2)R1 alleles in idiopathic membranous nephropathy. N Engl J Med 364:616–626
Beck LH Jr (2017) PLA2R and THSD7A: disparate paths to the same disease? J Am Soc Nephrol 28:2579–2589
Le WB, Shi JS, Zhang T, Liu L, Qin HZ, Liang S, Zhang YW, Zheng CX, Jiang S, Qin WS, Zhang HT, Liu ZH (2017) HLA-DRB1*15:01 and HLA-DRB3*02:02 in PLA2R-related membranous nephropathy. J Am Soc Nephrol 28:1642–1650
Cui Z, Xie LJ, Chen FJ, Pei ZY, Zhang LJ, Qu Z, Huang J, Gu QH, Zhang YM, Wang X, Wang F, Meng LQ, Liu G, Zhou XJ, Zhu L, Lv JC, Liu F, Zhang H, Liao YH, Lai LH, Ronco P, Zhao MH (2017) MHC class II risk alleles and amino acid residues in idiopathic membranous nephropathy. J Am Soc Nephrol 28:1651–1664
Wang HY, Cui Z, Xie LJ, Zhang LJ, Pei ZY, Chen FJ, Qu Z, Huang J, Zhang YM, Wang X, Wang F, Meng LQ, Cheng XY, Liu G, Zhou XJ, Zhang H, Debiec H, Ronco P, Zhao MH (2018) HLA class II alleles differing by a single amino acid associate with clinical phenotype and outcome in patients with primary membranous nephropathy. Kidney Int 94:974–982
Fresquet M, Jowitt TA, Gummadova J, Collins R, O'Cualain R, McKenzie EA, Lennon R, Brenchley PE (2015) Identification of a major epitope recognized by PLA2R autoantibodies in primary membranous nephropathy. J Am Soc Nephrol 26:302–313
Seitz-Polski B, Dolla G, Payre C, Girard CA, Polidori J, Zorzi K, Birgy-Barelli E, Jullien P, Courivaud C, Krummel T, Benzaken S, Bernard G, Burtey S, Mariat C, Esnault VL, Lambeau G (2016) Epitope spreading of autoantibody response to PLA2R associates with poor prognosis in membranous nephropathy. J Am Soc Nephrol 27:1517–1533
Seitz-Polski B, Debiec H, Rousseau A, Dahan K, Zaghrini C, Payre C, Esnault VLM, Lambeau G, Ronco P (2018) Phospholipase A2 receptor 1 epitope spreading at baseline predicts reduced likelihood of remission of membranous nephropathy. J Am Soc Nephrol 29:401–408
Seitz-Polski B, Dahan K, Debiec H, Rousseau A, Andreani M, Zaghrini C, Ticchioni M, Rosenthal A, Benzaken S, Bernard G, Lambeau G, Ronco P, Esnault VLM (2019) High-dose rituximab and early remission in PLA2R1-related membranous nephropathy. Clin J Am Soc Nephrol 14:1173–1182
Tomas NM, Beck LH Jr, Meyer-Schwesinger C, Seitz-Polski B, Ma H, Zahner G, Dolla G, Hoxha E, Helmchen U, Dabert-Gay AS, Debayle D, Merchant M, Klein J, Salant DJ, Stahl RAK, Lambeau G (2014) Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N Engl J Med 371:2277–2287
Stoddard SV, Welsh CL, Palopoli MM, Stoddard SD, Aramandla MP, Patel RM, Ma H, Beck LH Jr (2019) Structure and function insights garnered from in silico modeling of the thrombospondin type-1 domain-containing 7A antigen. Proteins 87:136–145
Herwig J, Skuza S, Sachs W, Sachs M, Failla AV, Rune G, Meyer TN, Fester L, Meyer-Schwesinger C (2019) Thrombospondin type 1 domain-containing 7A localizes to the slit diaphragm and stabilizes membrane dynamics of fully differentiated podocytes. J Am Soc Nephrol 30:824–839
Tomas NM, Hoxha E, Reinicke AT, Fester L, Helmchen U, Gerth J, Bachmann F, Budde K, Koch-Nolte F, Zahner G, Rune G, Lambeau G, Meyer-Schwesinger C, Stahl RA (2016) Autoantibodies against thrombospondin type 1 domain-containing 7A induce membranous nephropathy. J Clin Invest 126:2519–2532
Tomas NM, Meyer-Schwesinger C, von Spiegel H, Kotb AM, Zahner G, Hoxha E, Helmchen U, Endlich N, Koch-Nolte F, Stahl RAK (2017) A heterologous model of thrombospondin type 1 domain-containing 7A-associated membranous nephropathy. J Am Soc Nephrol 28:3262–3277
Seifert L, Hoxha E, Eichhoff AM, Zahner G, Dehde S, Reinhard L, Koch-Nolte F, Stahl RAK, Tomas NM (2018) The most N-terminal region of THSD7A is the predominant target for autoimmunity in THSD7A-associated membranous nephropathy. J Am Soc Nephrol 29:1536–1548
Fresquet M, Rhoden SJ, Jowitt TA, McKenzie EA, Roberts I, Lennon R, Brenchley PE (2019) Autoantigens PLA2R and THSD7A in membranous nephropathy share a common epitope motif in the N-terminal domain. J Autoimmun. https://doi.org/10.1016/j.jaut.2019102308
Hoxha E, Beck LH Jr, Wiech T, Tomas NM, Probst C, Mindorf S, Meyer-Schwesinger C, Zahner G, Stahl PR, Schopper R, Panzer U, Harendza S, Helmchen U, Salant DJ, Stahl RA (2017) An indirect immunofluorescence method facilitates detection of thrombospondin type 1 domain-containing 7A-specific antibodies in membranous nephropathy. J Am Soc Nephrol 28:520–531
De Vriese AS, Glassock RJ, Nath KA, Sethi S, Fervenza FC (2017) A proposal for a serology-based approach to membranous nephropathy. J Am Soc Nephrol 28:421–430
Cossey LN, Walker PD, Larsen CP (2013) Phospholipase A2 receptor staining in pediatric idiopathic membranous glomerulopathy. Pediatr Nephrol 28:2307–2311
Kumar V, Varma AK, Nada R, Ghosh R, Suri D, Gupta A, Kumar V, Rathi M, Kohli H, Jha V, Gupta K, Ramachandran R (2017) Primary membranous nephropathy in adolescence: a prospective study. Nephrology (Carlton) 22:678–683
Kanda S, Horita S, Yanagihara T, Shimizu A, Hattori M (2017) M-type phospholipase A2 receptor (PLA2R) glomerular staining in pediatric idiopathic membranous nephropathy. Pediatr Nephrol 32:713–717
Zhang D, Wu Y, Zhang C, Zhang W, Zou J, Jiang G (2019) Compared staining of the phospholipase A2 receptor in the glomeruli of Chinese adults and children with idiopathic membranous nephropathy. Pathol Res Pract 215:952–956
Dettmar AK, Wiech T, Kemper MJ, Soave A, Rink M, Oh J, Stahl RAK, Hoxha E, Pediatric MN Study Group (2018) Immunohistochemical and serological characterization of membranous nephropathy in children and adolescents. Pediatr Nephrol 33:463–472
Al-Rabadi L, Ayalon R, Bonegio RG, Ballard JE, Fujii AM, Henderson JM, Salant DJ, Beck LH Jr (2016) Pregnancy in a patient with primary membranous nephropathy and circulating anti-PLA2R antibodies: a case report. Am J Kidney Dis 67:775–778
Sachdeva M, Sheikh F, Beck LH, Fishbane S, Miller LJ (2018) Transplacental passage of phospholipase A2 receptor antibodies from maternal to fetal circulation and secretion into breastmilk. J Am Soc Nephrol 29:379
Leon J, Perez-Saez MJ, Batal I, Beck LH Jr, Rennke HG, Canaud G, Legendre C, Pascual J, Riella LV (2019) Membranous nephropathy post-transplantation: an update of the pathophysiology and management. Transplantation. https://doi.org/10.1097/TP.0000000000002758
Grupper A, Cornell LD, Fervenza FC, Beck LH Jr, Lorenz E, Cosio FG (2016) Recurrent membranous nephropathy after kidney transplantation: treatment and long-term implications. Transplantation 100:2710–2716
Cameron JS (1990) Membranous nephropathy in childhood and its treatment. Pediatr Nephrol 4:193–198
Trautmann A, Lipska-Zietkiewicz BS, Schaefer F (2018) Exploring the clinical and genetic Spectrum of steroid resistant nephrotic syndrome: the PodoNet Registry. Front Pediatr 6:200
KDIGO (2012) KDIGO clinical practice guideline for glomerulonephritis. Kidney Int Suppl:139–274
Sethi S, Debiec H, Madden B, Charlesworth MC, Morelle J, Gross LA, Ravindran A, Buob D, Jadoul M, Fervenza FC, Ronco P (2019) Neural epidermal growth factor-like 1 protein (NELL-1) associated membranous nephropathy. Kidney Int. https://doi.org/10.1016/j.kint.2019.09.014
Hoxha E, Harendza S, Pinnschmidt HO, Tomas NM, Helmchen U, Panzer U, Stahl RA (2015) Spontaneous remission of proteinuria is a frequent event in phospholipase A2 receptor antibody-negative patients with membranous nephropathy. Nephrol Dial Transplant 30:1862–1869
Polanco N, Gutierrez E, Covarsi A, Ariza F, Carreno A, Vigil A, Baltar J, Fernandez-Fresnedo G, Martin C, Pons S, Lorenzo D, Bernis C, Arrizabalaga P, Fernandez-Juarez G, Barrio V, Sierra M, Castellanos I, Espinosa M, Rivera F, Oliet A, Fernandez-Vega F, Praga M, Grupo de Estudio de las Enfermedades Glomerulares de la Sociedad Española de Nefrología (2010) Spontaneous remission of nephrotic syndrome in idiopathic membranous nephropathy. J Am Soc Nephrol 21:697–704
Cattran DC, Delmore T, Roscoe J, Cole E, Cardella C, Charron R, Ritchie S (1989) A randomized controlled trial of prednisone in patients with idiopathic membranous nephropathy. N Engl J Med 320:210–215
Valentini RP, Mattoo TK, Kapur G, Imam A (2009) Membranous glomerulonephritis: treatment response and outcome in children. Pediatr Nephrol 24:301–308
Arif MK, Arif M, Amjad N (2016) A histopathological outlook on nephrotic syndrome: a pediatric perspective. Indian J Nephrol 26:188–191
Remuzzi G, Chiurchiu C, Abbate M, Brusegan V, Bontempelli M, Ruggenenti P (2002) Rituximab for idiopathic membranous nephropathy. Lancet 360:923–924
Ruggenenti P, Cravedi P, Chianca A, Perna A, Ruggiero B, Gaspari F, Rambaldi A, Marasa M, Remuzzi G (2012) Rituximab in idiopathic membranous nephropathy. J Am Soc Nephrol 23:1416–1425
Fervenza FC, Cosio FG, Erickson SB, Specks U, Herzenberg AM, Dillon JJ, Leung N, Cohen IM, Wochos DN, Bergstralh E, Hladunewich M, Cattran DC (2008) Rituximab treatment of idiopathic membranous nephropathy. Kidney Int 73:117–125
Fervenza FC, Abraham RS, Erickson SB, Irazabal MV, Eirin A, Specks U, Nachman PH, Bergstralh EJ, Leung N, Cosio FG, Hogan MC, Dillon JJ, Hickson LJ, Li X, Cattran DC, Mayo Nephrology Collaborative Group (2010) Rituximab therapy in idiopathic membranous nephropathy: a 2-year study. Clin J Am Soc Nephrol 5:2188–2198
Dahan K, Debiec H, Plaisier E, Cachanado M, Rousseau A, Wakselman L, Michel PA, Mihout F, Dussol B, Matignon M, Mousson C, Simon T, Ronco P, GEMRITUX Study Group (2017) Rituximab for severe membranous nephropathy: a 6-month trial with extended follow-up. J Am Soc Nephrol 28:348–358
Fervenza FC, Appel GB, Barbour SJ, Rovin BH, Lafayette RA, Aslam N, Jefferson JA, Gipson PE, Rizk DV, Sedor JR, Simon JF, McCarthy ET, Brenchley P, Sethi S, Avila-Casado C, Beanlands H, Lieske JC, Philibert D, Li T, Thomas LF, Green DF, Juncos LA, Beara-Lasic L, Blumenthal SS, Sussman AN, Erickson SB, Hladunewich M, Canetta PA, Hebert LA, Leung N, Radhakrishnan J, Reich HN, Parikh SV, Gipson DS, Lee DK, da Costa BR, Juni P, Cattran DC, Investigators MENTOR (2019) Rituximab or cyclosporine in the treatment of membranous nephropathy. N Engl J Med 381:36–46
Sequential therapy with tacrolimus and rituximab in primary membranous nephropathy (STARMEN). https://clinicaltrials.gov/ct2/show/NCT01955187 Accessed 26 Nov. 2019
Rituximab versus steroids and cyclophosphamide in the treatment of idiopathic membranous nephropathy (RI-CYCLO). https://clinicaltrials.gov/ct2/show/NCT03018535 Accessed 26 Nov. 2019
Iijima K, Sako M, Nozu K, Mori R, Tuchida N, Kamei K, Miura K, Aya K, Nakanishi K, Ohtomo Y, Takahashi S, Tanaka R, Kaito H, Nakamura H, Ishikura K, Ito S, Ohashi Y, Rituximab for Childhood-onset Refractory Nephrotic Syndrome Study Group (2014) Rituximab for childhood-onset, complicated, frequently relapsing nephrotic syndrome or steroid-dependent nephrotic syndrome: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet 384:1273–1281
Sun L, Xu H, Shen Q, Cao Q, Rao J, Liu HM, Fang XY, Zhou LJ (2014) Efficacy of rituximab therapy in children with refractory nephrotic syndrome: a prospective observational study in Shanghai. World J Pediatr 10:59–63
Ravani P, Rossi R, Bonanni A, Quinn RR, Sica F, Bodria M, Pasini A, Montini G, Edefonti A, Belingheri M, De Giovanni D, Barbano G, Degl'Innocenti L, Scolari F, Murer L, Reiser J, Fornoni A, Ghiggeri GM (2015) Rituximab in children with steroid-dependent nephrotic syndrome: a multicenter, open-label, noninferiority, randomized controlled trial. J Am Soc Nephrol 26:2259–2266
Malatesta-Muncher R, Eldin KW, Beck LH Jr, Michael M (2018) Idiopathic membranous nephropathy in children treated with rituximab: report of two cases. Pediatr Nephrol 33:1089–1092
James KE, Xiao R, Merkel PA, Weiss PF (2017) Variation in the treatment of children hospitalized with antineutrophil cytoplasmic antibody-associated vasculitis in the US. Arthritis Care Res 69:1377–1383
Jariwala MP, Laxer RM (2018) Primary vasculitis in childhood: GPA and MPA in childhood. Front Pediatr 6:226
Kallash M, Smoyer WE, Mahan JD (2019) Rituximab use in the management of childhood nephrotic syndrome. Front Pediatr 7:178
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Drs. Safar-Boueri, Piya, and Ayalon have nothing to disclose. Dr. Beck discloses the following: Coinventor on the patent “Diagnostics for membranous nephropathy” with royalties through Boston University; royalties from UpToDate for topic cards on membranous nephropathy; grant support from Sanofi/Genzyme. Advisory board participant for Genentech, Visterra.
Additional information
Answers: 1. c; 2. b; 3. b; 4. a; 5. c
Key summary points
• Membranous nephropathy should always be considered in the differential diagnosis of childhood and adolescent nephrotic syndrome
• Two main antigens (PLA2R and THSD7A) have been found to be the targets of autoimmunity in both pediatric and adult disease
• Detection and monitoring of circulating autoantibodies, which precede and predict clinical disease activity, are important for diagnosis and the monitoring of immunologic disease status in membranous nephropathy
• Proteinuria may continue well beyond the cessation of immunologic disease activity, and therefore does not always require initiation or escalation of immunosuppressive therapy.
• As in adult disease, the B cell-depleting agent rituximab may be a useful agent for the treatment of pediatric MN.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Safar-Boueri, L., Piya, A., Beck, L.H. et al. Membranous nephropathy: diagnosis, treatment, and monitoring in the post-PLA2R era. Pediatr Nephrol 36, 19–30 (2021). https://doi.org/10.1007/s00467-019-04425-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-019-04425-1