Abstract
Background
Strategies to expand numbers of deceased donor kidneys suitable for pediatric recipients are urgently needed to prevent long-term dialysis-associated morbidity and mortality. Donors designated as increased risk of disease transmission (IRD) are infrequently used in pediatric recipients. We examined outcomes of these kidneys in pediatric patients and the potential to increase the donor pool.
Methods
The United Network for Organ Sharing (UNOS) database records presence of IRD in all deceased donors since 2004. All pediatric kidney transplant recipients from 2004 to 2017 were identified and stratified by IRD status, and outcomes were examined.
Results
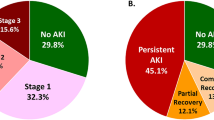
Four hundred seventy-three pediatric kidney transplant recipients received an IRD allograft. IRD donors had lower kidney donor profile index (KDPI); were more likely to be younger, male, and Caucasian; and were more likely to have used drugs. IRD kidneys were more likely to have been biopsied and placed on pulsatile perfusion. Other than an older recipient age, demographic data were not different between groups. Allograft and patient survivals were similar, as were rejection and delayed graft function rates. Compared with adult recipients and adult IRD recipients, pediatric recipients were more likely to have a younger donor, receive a kidney with a lower creatinine, and were less likely to have delayed graft function (p < 0.05). There were no recorded disease transmissions in IRD group.
Conclusions
Patient and allograft survivals are similar in IRD and non-IRD kidneys. High-quality IRD organs used in adults represent a large number of donors with excellent outcomes. IRD allografts have a potential to increase transplant volume and should be considered for pediatric patients.


Similar content being viewed by others
References
Hart A, Smith JM, Skeans MA, Gustafson SK, Wilk AR, Robinson A, Wainright JL, Haynes CR, Snyder JJ, Kasiske BL, Israni AK (2018) OPTN/SRTR 2016 annual data report: kidney. Am J Transplant 18(Suppl 1):18–113. https://doi.org/10.1111/ajt.14557
Seem DL, Lee I, Umscheid CA, Kuehnert MJ, United States Public Health Service (2013) PHS guideline for reducing human immunodeficiency virus, hepatitis B virus, and hepatitis C virus transmission through organ transplantation. Public Health Rep 128:247–343. https://doi.org/10.1177/003335491312800403
Public comment proposal: guidance on explaining risk related to use of U.S. PHS increased risk donor organs when considering organ offers. OPTN/UNOS Ad-hoc Disease Transmission Advisory Committee
Kucirka LM, Singer AL, Segev DL (2011) High infectious risk donors: what are the risks and when are they too high? Curr Opin Organ Transplant 16:256–261. https://doi.org/10.1097/MOT.0b013e3283449dd3
Wrenn SM, Callas PW, Kapoor T, Aunchman AF, Paine AN, Pineda JA, Marroquin CE (2017) Increased risk organ transplantation in the pediatric population. Pediatr Transplant 21(8). https://doi.org/10.1111/petr.13041
Morrison AK, Gowda C, Tumin D, Phelps CM, Hayes D Jr, Tobias J, Gajarski RJ, Nandi D (2018) Pediatric marginal donor hearts: trends in US national use, 2005-2014. Pediatr Transplant 22:e13216. https://doi.org/10.1111/petr.13216
Khan AM, Green RS, Lytrivi ID, Sahulee R (2016) Donor predictors of allograft utilization for pediatric heart transplantation. Transpl Int 29:1269–1275. https://doi.org/10.1111/tri.12835
Sahulee R, Lytrivi ID, Savla JJ, Rossano JW (2014) Centers for Disease Control “high-risk” donor status does not significantly affect recipient outcome after heart transplantation in children. J Heart Lung Transplant 33:1173–1177. https://doi.org/10.1016/j.healun.2014.06.005
Kucirka LM, Ros RL, Subramanian AK, Montgomery RA, Segev DL (2011) Provider response to a rare but highly publicized transmission of HIV through solid organ transplantation. Arch Surg 146:41–45. https://doi.org/10.1001/archsurg.2010.303
Irwin L, Kotton CN, Elias N, Palafox J, Basler D, Shao SH, Lester W, Zhang X, Kimball B, Trencher C, Fishman JA (2017) Utilization of increased risk for transmission of infectious disease donor organs in solid organ transplantation: retrospective analysis of disease transmission and safety. Transpl Infect Dis 19(6). https://doi.org/10.1111/tid.12791
Tsiouris A, Wilson L, Sekar RB, Mangi AA, Yun JJ (2016) Heart transplant outcomes in recipients of Centers for Disease Control (CDC) high risk donors. J Card Surg 31:772–777. https://doi.org/10.1111/jocs.12861
Gaffey AC, Doll SL, Thomasson AM, Venkataraman C, Chen CW, Goldberg LR, Blumberg EA, Acker MA, Stone F, Atluri P (2016) Transplantation of “high-risk” donor hearts: implications for infection. J Thorac Cardiovasc Surg 152:213–220. https://doi.org/10.1016/j.jtcvs.2015.12.062
Hwang CS, Levea SL, Parekh JR, Liang Y, Desai DM, MacConmara M (2019) Should more donation after cardiac death livers be used in pediatric transplantation? Pediatr Transplant 23:e13323. https://doi.org/10.1111/petr.13323
Duan KI, Englesbe MJ, Volk ML (2010) Centers for Disease Control ‘high-risk’ donors and kidney utilization. Am J Transplant 10:416–420. https://doi.org/10.1111/j.1600-6143.2009.02931.x
Author information
Authors and Affiliations
Contributions
Christine S. Hwang: first author, gathered, organized, and analyzed the data and wrote the manuscript.
Jyothsna Gattineni: participated in reviewing the manuscript.
Malcolm MacConmara: participated in gathering, organizing, and performing data analysis and writing the manuscript.
All authors have approved the manuscript as submitted.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Disclaimer
The data reported here were supplied by the Minneapolis Medical Research Foundation as the contractor for the SRTR. The interpretation and reporting of these data are the responsibility of the author(s) and in no way, should be an official policy of or interpretation by the SRTR or the US government.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hwang, C.S., Gattineni, J. & MacConmara, M. Utilizing increased risk for disease transmission (IRD) kidneys for pediatric renal transplant recipients. Pediatr Nephrol 34, 1743–1751 (2019). https://doi.org/10.1007/s00467-019-04276-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-019-04276-w