Abstract
Background
Heterozygous PAX2 mutations cause renal coloboma syndrome (RCS) [OMIM no. 120330]. RCS is a renal syndromic disease encompassing retinal coloboma and sensorineural hearing loss. Recently, a causative role for PAX2 was reported in adult-onset nephrotic syndrome secondary to focal segmental glomerulosclerosis (FSGS). However, the prevalence of PAX2 mutations among large cohort of children with steroid-resistant nephrotic syndrome (SRNS) and FSGS has not been systematically studied.
Methods
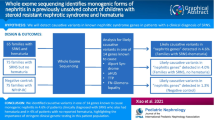
We employed whole-exome sequencing (WES) to identify the percentage of SRNS cases explained by monogenic mutations in known genes of SRNS/FSGS. As PAX2 mutations are not an established cause of childhood FSGS, we evaluated a cohort of 215 unrelated families with SRNS, in whom no underlying genetic etiology had been previously established.
Results
Using WES, we identified 3 novel causative heterozygous PAX2 mutations in 3 out of the 215 unrelated index cases studied (1.3%). All three cases were detected in individuals from families with more than one affected and compatible with an autosomal dominant mode of inheritance (3/57 familial cases studied (5.2%)). The clinical diagnosis in three out of four pediatric index patients was done during routine medical evaluation.
Conclusions
Our findings demonstrate high frequency of PAX2 mutations in familial form of SRNS (5.2%) and further expand the phenotypic spectrum of PAX2 heterozygous mutations to include autosomal dominant childhood-onset FSGS. These results highlight the importance of including PAX2 in the list of genes known to cause FSGS in children.

Similar content being viewed by others
References
Sanyanusin P, Schimmenti LA, McNoe LA, Ward TA, Pierpont ME, Sullivan MJ, Dobyns WB, Eccles MR (1995) Mutation of the PAX2 gene in a family with optic nerve colobomas, renal anomalies and vesicoureteral reflux. Nat Genet 9:358–364
Weber S, Moriniere V, Knuppel T, Charbit M, Dusek J, Ghiggeri GM, Jankauskiene A, Mir S, Montini G, Peco-Antic A, Wuhl E, Zurowska AM, Mehls O, Antignac C, Schaefer F, Salomon R (2006) Prevalence of mutations in renal developmental genes in children with renal hypodysplasia: results of the ESCAPE study. J Am Soc Nephrol: JASN 17:2864–2870
Bower M, Salomon R, Allanson J, Antignac C, Benedicenti F, Benetti E, Binenbaum G, Jensen UB, Cochat P, DeCramer S, Dixon J, Drouin R, Falk MJ, Feret H, Gise R, Hunter A, Johnson K, Kumar R, Lavocat MP, Martin L, Moriniere V, Mowat D, Murer L, Nguyen HT, Peretz-Amit G, Pierce E, Place E, Rodig N, Salerno A, Sastry S, Sato T, Sayer JA, Schaafsma GC, Shoemaker L, Stockton DW, Tan WH, Tenconi R, Vanhille P, Vats A, Wang X, Warman B, Weleber RG, White SM, Wilson-Brackett C, Zand DJ, Eccles M, Schimmenti LA, Heidet L (2012) Update of PAX2 mutations in renal coloboma syndrome and establishment of a locus-specific database. Hum Mutat 33:457–466
Bower MA, Schimmenti LA, Eccles MR (1993) PAX2-Related Disorder. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, Amemiya A (eds) GeneReviews((R)), Seattle (WA)
Barua M, Stellacci E, Stella L, Weins A, Genovese G, Muto V, Caputo V, Toka HR, Charoonratana VT, Tartaglia M, Pollak MR (2014) Mutations in PAX2 associate with adult-onset FSGS. J Am Soc Nephrol: JASN 25:1942–1953
Okumura T, Furuichi K, Higashide T, Sakurai M, Hashimoto S, Shinozaki Y, Hara A, Iwata Y, Sakai N, Sugiyama K, Kaneko S, Wada T (2015) Association of PAX2 and other gene mutations with the clinical manifestations of renal coloboma syndrome. PLoS One 10:e0142843
Vivante A, Hildebrandt F (2016) Exploring the genetic basis of early-onset chronic kidney disease. Nat Rev Nephrol 12:133–146
Lovric S, Ashraf S, Tan W, Hildebrandt F (2016) Genetic testing in steroid-resistant nephrotic syndrome: when and how? Nephrol Dial Transplant 31(11):1802–1813
Sadowski CE, Lovric S, Ashraf S, Pabst WL, Gee HY, Kohl S, Engelmann S, Vega-Warner V, Fang H, Halbritter J, Somers MJ, Tan W, Shril S, Fessi I, Lifton RP, Bockenhauer D, El-Desoky S, Kari JA, Zenker M, Kemper MJ, Mueller D, Fathy HM, Soliman NA, Hildebrandt F (2015) A single-gene cause in 29.5% of cases of steroid-resistant nephrotic syndrome. J Am Soc Nephrol: JASN 26:1279–1289
Warejko JK, Tan W, Daga A, Schapiro D, Lawson JA, Shril S, Lovric S, Ashraf S, Rao J, Hermle T, Jobst-Schwan T, Widmeier E, Majmundar AJ, Schneider R, Gee HY, Schmidt JM, Vivante A, van der Ven AT, Ityel H, Chen J, Sadowski CE, Kohl S, Pabst WL, Nakayama M, Somers MJG, Rodig NM, Daouk G, Baum M, Stein DR, Ferguson MA, Traum AZ, Soliman NA, Kari JA, El Desoky S, Fathy H, Zenker M, Bakkaloglu SA, Muller D, Noyan A, Ozaltin F, Cadnapaphornchai MA, Hashmi S, Hopcian J, Kopp JB, Benador N, Bockenhauer D, Bogdanovic R, Stajic N, Chernin G, Ettenger R, Fehrenbach H, Kemper M, Munarriz RL, Podracka L, Buscher R, Serdaroglu E, Tasic V, Mane S, Lifton RP, Braun DA, Hildebrandt F (2018) Whole exome sequencing of patients with steroid-resistant nephrotic syndrome. Clin J Am Soc Nephrol : CJASN 13:53–62
Vivante A, Hwang DY, Kohl S, Chen J, Shril S, Schulz J, van der Ven A, Daouk G, Soliman NA, Kumar AS, Senguttuvan P, Kehinde EO, Tasic V, Hildebrandt F (2017) Exome sequencing discerns syndromes in patients from consanguineous families with congenital anomalies of the kidneys and urinary tract. J Am Soc Nephrol 28:69–75
Vivante A, Ityel H, Pode-Shakked B, Chen J, Shril S, van der Ven AT, Mann N, Schmidt JM, Segel R, Aran A, Zeharia A, Staretz-Chacham O, Bar-Yosef O, Raas-Rothschild A, Landau YE, Lifton RP, Anikster Y, Hildebrandt F (2017) Exome sequencing in Jewish and Arab patients with rhabdomyolysis reveals single-gene etiology in 43% of cases. Pediatr Nephrol 32:2273–2282
Vivante A, Mark-Danieli M, Davidovits M, Harari-Steinberg O, Omer D, Gnatek Y, Cleper R, Landau D, Kovalski Y, Weissman I, Eisenstein I, Soudack M, Wolf HR, Issler N, Lotan D, Anikster Y, Dekel B (2013) Renal hypodysplasia associates with a WNT4 variant that causes aberrant canonical WNT signaling. J Am Soc Nephrol 24:550–558
Sanyanusin P, McNoe LA, Sullivan MJ, Weaver RG, Eccles MR (1995) Mutation of PAX2 in two siblings with renal-coloboma syndrome. Hum Mol Genet 4:2183–2184
Cheong HI, Cho HY, Kim JH, Yu YS, Ha IS, Choi Y (2007) A clinico-genetic study of renal coloboma syndrome in children. Pediatr Nephrol 22:1283–1289
Amiel J, Audollent S, Joly D, Dureau P, Salomon R, Tellier AL, Auge J, Bouissou F, Antignac C, Gubler MC, Eccles MR, Munnich A, Vekemans M, Lyonnet S, Attie-Bitach T (2000) PAX2 mutations in renal-coloboma syndrome: mutational hotspot and germline mosaicism. Eur J Hum Genet : EJHG 8:820–826
Favor J, Sandulache R, Neuhauser-Klaus A, Pretsch W, Chatterjee B, Senft E, Wurst W, Blanquet V, Grimes P, Sporle R, Schughart K (1996) The mouse Pax2(1Neu) mutation is identical to a human PAX2 mutation in a family with renal-coloboma syndrome and results in developmental defects of the brain, ear, eye, and kidney. Proc Natl Acad Sci U S A 93:13870–13875
Dressler GR (2011) Patterning and early cell lineage decisions in the developing kidney: the role of Pax genes. Pediatr Nephrol 26:1387–1394
Naiman N, Fujioka K, Fujino M, Valerius MT, Potter SS, McMahon AP, Kobayashi A (2017) Repression of interstitial identity in nephron progenitor cells by Pax2 establishes the nephron-Interstitium boundary during kidney development. Dev Cell 41:349–365 e343
Wagner KD, Wagner N, Guo JK, Elger M, Dallman MJ, Bugeon L, Schedl A (2006) An inducible mouse model for PAX2-dependent glomerular disease: insights into a complex pathogenesis. Curr Biol 16:793–800
Dressler GR, Wilkinson JE, Rothenpieler UW, Patterson LT, Williams-Simons L, Westphal H (1993) Deregulation of Pax-2 expression in transgenic mice generates severe kidney abnormalities. Nature 362:65–67
Funding
F.H. the William E. Harmon Professor of Pediatrics was supported by a grant from the National Institutes of Health to FH (R01-DK076683). A.V. is a recipient of the Fulbright Post-doctoral Scholar Award for 2013. A.V. is also supported by grants from the Manton Center Fellowship program, Boston Children’s Hospital, Boston, MA and the Mallinckrodt Research Fellowship Award. This work was also supported by German Federal Ministry for Education and Research (BMBF) Grant STOP-FSGS 01GM1518A (to M. S.). Sequencing and data processing was performed by the Broad and Yale Centers for Mendelian Genomics funded by the National Human Genome Research Institute (UM1 HG008900 to DGM and HLR and U54 HG006504 to RPL).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved by the Institutional Review Board of the University of Michigan and Boston Children’s Hospital.
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vivante, A., Chacham, O.S., Shril, S. et al. Dominant PAX2 mutations may cause steroid-resistant nephrotic syndrome and FSGS in children. Pediatr Nephrol 34, 1607–1613 (2019). https://doi.org/10.1007/s00467-019-04256-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-019-04256-0