Abstract
Background
In neonates, the validation of urinary biomarkers to diagnose acute kidney injury is a rapidly evolving field. The neonatal population poses unique challenges when assessing the collection, storage, and processing of urinary samples for biomarker analysis. Given this, establishing optimal and consistent sample processing in this population for meaningful use in ongoing clinical trials is important.
Methods
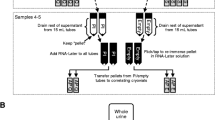
Urine from a cohort of 19 hospitalized neonatal intensive care unit patients enrolled in the Preterm Erythropoietin Neuroprotection Trial (Clinical Trial NCT01378273) was collected for biomarker analysis by indirect techniques using Fisher-brand cotton balls placed in the diapers. Fourteen urinary biomarkers were measured using commercially available kits via electrochemiluminescence on multiarray plates and compared between paired samples processed with centrifugation prior to storage versus prior to analysis.
Results
None of the biomarker concentrations differed between samples undergoing centrifugation prior to storage versus prior to analysis. The difference between samples was within 2% of the estimated concentration for the protein in 12 of 14 biomarkers (86%), and all paired biomarker concentrations were within 4%. The percentage error analysis did not show a difference between paired samples, with biomarker percentage errors smaller than the stated immunoassay coefficient of variance.
Conclusions
The urinary concentrations of biomarkers were comparable between paired samples, demonstrating that indirectly collected neonatal urine samples do not require centrifugation after collection and before storage. The ability to use routine urine collection and storage methods to obtain samples for subsequent quantitative immunoassay analysis should facilitate studies of newborns and young children.


Similar content being viewed by others
References
Askenazi DJ, Koralkar R, Patil N, Halloran B, Ambalavanan N, Griffin R (2016) Acute kidney injury urine biomarkers in very low-birth-weight infants. Clin J Am Soc Nephrol 11:1527–1535
Sohn AH, Garrett DO, Sinkowitz-Cochran RL, Grohskopf LA, Levine GL, Stover BH, Jarvis WR, Network PP (2001) Prevalence of nosocomial infections in neonatal intensive care unit patients: results from the first national point-prevalence survey. J Pediatr 139:821–827
Muratore C, Dhanireddy R (1993) Urine collection for disposable diapers in premature infants: biochemical analysis. Clin Pediatr (Phila) 32:314–315
Starr R, Kimmel P, Bonventre J. Best practices for sample storage: a report from the Workshop on Urine Biospecimen Handling [Internet]. http://www3.niddk.nih.gov/fund/other/Best_Practices_for_sample_Storage.pdf
Parikh CR, Butrymowicz I, Yu A, Chinchilli VM, Park M, Hsu CY, Reeves WB, Dervarjan P, Kimmel PL, Siew ED, Liu KD, ASSESS-AKI Study Investigators (2014) Urine stability studies for novel biomarkers of acute kidney injury. Am J Kidney Dis 63:567–572
Schuh MP, Nehus E, Ma Q, Haffner C, Bennett M, Krawczeski CD, Devarajan P (2016) Long-term stability of urinary biomarkers of acute kidney injury in children. Am J Kidney Dis 67:56–61
Mock DM (1992) Rayon balls and disposable-diaper material selectively adsorb creatinine. Am J Clin Nutr 55:326–330
Juul SE, Mayock DE, Comstock BA, Heagerty PJ (2015) Neuroprotective potential of erythropoietin in neonates; design of a randomized trial. Matern Health Neonatol Perinatol 1:27
Meso Scale Discovery (MSD). Kidney Injury Panel 3 (human) Kit: MDS toxicology assays [Internet]. MSD, Gaithersburg. https://www.mesoscale.com/~/media/files/product%20inserts/kidney%20injury%20panel%203%20human.pdf. Accessed Jan 2017
Meso Scale Discovery (MSD). Kidney Injury Panel 5 (human) Kit: MDS Multi-Spot Assay System [Internet]. MSD, Gaithersburg. https://www.mesoscale.com/~/media/files/product%20inserts/kidney%20injury%20panel%205%20human.pdf. Accessed Jan 2017
R Core Team (2013). R: A language and environment for statistical computing. R Foundations for Statistical Computing, Vienna. https://www.R-project.org
Hochberg Y (1988) A sharper bonferroni procedure for multiple tests of significance. Biometrika 75:800
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1:307–310
Hofman CS, Melis RJ, Donders AR (2015) Adapted Bland–Altman method was used to compare measurement methods with unequal observations per case. J Clin Epidemiol 68:939–943
Bland JM, Altman DG (1995) Comparing methods of measurement: why plotting difference against standard method is misleading. Lancet 346:1085–1087
Giesen C, Lieske JC (2016) The influence of processing and storage conditions on renal protein biomarkers. Clin J Am Soc Nephrol 11:1726–1728
Beeram MR, Dhanireddy R (1991) Urinalysis: direct versus diaper collection. Clin Pediatr (Phila) 30:278–280
Acknowledgments
We thank Elizabeth Howland, Amy Silvia, Stephanie Hauge, and Emily Pao for their assistance with this work. This study was supported by an internal grant from the Seattle Children’s Research Institute Center for Clinical and Translational Research (SH). It was also supported by the NIH R01 DK103608 (SH, DA, SG), U01NS077953 and NCT01378273 (SJ, DM), and T32DK007662 (MS). The authors declare that they have no other relevant financial interests.
Author information
Authors and Affiliations
Contributions
Research idea and study design: DA, SG, JM, PB, SJ, DM, SH; data acquisition: SJ, DM, SH; data analysis and interpretation: MS, DA, SG, JM, TB, ZA, PB, SH; statistical analysis: MS, JM, TB; supervision or mentorship: SH. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. SH takes responsibility that this study is reported honestly, accurately, and transparently; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Corresponding author
Ethics declarations
Informed consent was obtained as part of the PENUT study, and all study procedures were performed in accordance with ethical standards of the local Institutional Review Board and Good Clinical Practice guidelines.
Conflict of interest
None to declare.
Rights and permissions
About this article
Cite this article
Starr, M.C., Askenazi, D.J., Goldstein, S.L. et al. Impact of processing methods on urinary biomarkers analysis in neonates. Pediatr Nephrol 33, 181–186 (2018). https://doi.org/10.1007/s00467-017-3779-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-017-3779-0