Abstract
Background
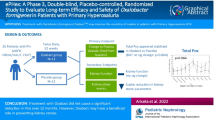
Primary hyperoxaluria (PH) is a rare, genetic disorder which involves the overproduction of endogenous oxalate, leading to hyperoxaluria, recurrent urolithiasis and/or progressive nephrocalcinosis and eventually resulting in kidney failure and systemic oxalosis. The aim of this trial was to investigate whether treatment involving an oxalate-metabolising bacterium (Oxalobacter formigenes) could reduce urinary oxalate excretion in PH patients.
Methods
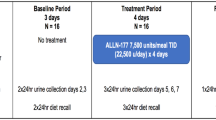
The efficacy and safety of O. formigenes (Oxabact® OC5; OxThera AB, Stockholm, Sweden) was evaluated in a randomised, placebo-controlled, double-blind study for 8 weeks. The primary objective was reduction in urinary oxalate excretion (Uox). Secondary objectives included faecal O. formigenes count and decrease in plasma oxalate concentration (Pox).
Results
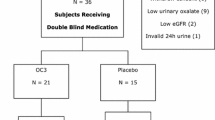
Twenty-eight patients randomised 1:1 to the treatment group (OC5) or the placebo group completed the study. After 8 weeks of treatment, there was no significant difference in the change in Uox (mmol/24 h/1.73 m2) between the groups (OC5: +0.042, placebo: −0.140). Post-hoc analysis showed a statistically significant increase in Uox per urinary creatinine excretion in the OC5 group (OC5: +5.41, placebo: −15.96; p = 0.030). Change in Pox from baseline was not significantly different between groups (p = 0.438). The O. formigenes cell count was significantly increased in OC5-treated patients (p < 0.001) versus placebo. The treatment response to O. formigenes was related to individual stage of kidney deterioration, and Pox was directly correlated to kidney function, even for early-stage patients (chronic kidney disease stage 1). No safety issues were observed.
Conclusions
Treatment with OC5 did not significantly reduce Uox or Pox over 8 weeks of treatment. The treatment was well tolerated and successfully delivered to the gastrointestinal tract.

Similar content being viewed by others
References
Cochat P, Rumsby G (2013) Primary hyperoxaluria. N Engl J Med 369:649–658
Danpure CJ, Jennings PR, Fryer P, Purdue PE, Allsop J (1994) Primary hyperoxaluria type 1: genotypic and phenotypic heterogeneity. J Inherit Metab Dis 17:487–499
Hoppe B, Beck B, Milliner D (2009) The primary hyperoxalurias. Kidney Int 75:1264–1271
Belostotsky R, Seboun E, Idelson Milliner DS, Becker-Cohen R, Rinat C, Monico CG, Feinstein S, Ben-Shalom E, Magen D, Weissman I, Charon C, Frishberg Y (2010) Mutations in DHDPSL are responsible for primary hyperoxaluria type III. Am J Hum Gen 87:392–399
Cochat P, Deloraine A, Rotily M, Olive F, Liponski I, Deries N (1995) Epidemiology of primary hyperoxaluria type 1. Nephrol Dial Transplant 10[Suppl 8]:3–7
Kopp N, Leumann E (1995) Changing pattern of primary hyperoxaluria in Switzerland. Nephrol Dial Transplant 10:2224–2227
van Woerden CS, Groothoff JW, Wanders RJ, Davin JC, Wijburg FA (2003) Primary hyperoxaluria type 1 in the Netherlands: prevalence and outcome. Nephrol Dial Transplant 18:273–279
Leumann E, Hoppe B (2001) The primary hyperoxalurias. J Am Soc Nephrol 12:1986–1993
Hopp K, Cogal AG, Bergstralh EJ, Seide BM, Olson JB, Meek AM, Lieske JC, Milliner DS, Harris PC, Rare Kidney Stone Consortium (2015) Phenotype-genotype correlations and estimated carrier frequencies of primary hyperoxaluria. J Am Soc Nephrol 26:2559–2570
Hoppe B (2012) An update on primary hyperoxaluria. Nat Rev Nephrol 8:467–475
Mulay SR, Kulkarni OP, Rupanagudi KV, Migliorini A, Darisipudi MN, Vilaysane A, Muruve D, Shi Y, Munro F, Liapis H, Anders HJ (2013) Calcium oxalate crystals induce renal inflammation by NLRP3-mediated IL-1β secretion. J Clin Invest 123:236–246
Worcester E, Evan A, Coe F, Lingeman J, Krambeck A, Sommers A, Phillips C, Milliner D (2013) A test of the hypothesis that oxalate secretion produces proximal tubule crystallization in primary hyperoxaluria type 1. Am J Physiol Renal Physiol 305:FI574–584
Tang X, Bergstralh EJ, Mehta RA, Vrtiska TJ, Milliner DS, Lieske JC (2015) Nephrocalcinosis is a risk factor for kidney failure in primary hyperoxaluria. Kidney Int 87:623–631
Zhao F, Bergstralh EJ, Mehta RA, Vaughan LE, Olson JB, Seide BM, Meek AM, Cogal AG, Lieske JC, Milliner DS, Investigators of the Rare Kidney Stone Consortium (2016) Predictors of incident ESRD among patients with primary hyperoxaluria presenting prior to kidney failure. Clin J Am Soc Nephrol 11:119–126
Lieske JC, Monico CG, Holmes WS, Bergstralh EJ, Slezak JM, Rohlinger AL, Olson JB, Milliner DS (2005) International registry for Primary Hyperoxaluria. Am J Nephrol 25:290–296
Harambat J, Fargue S, Acquaviva C, Gagnadoux MF, Janssen F, Liutkus A, Mourani C, Macher MA, Abramowicz D, Legendre C, Durrbach A, Tsimaratos M, Nivet H, Girardin E, Schott AM, Rolland MO, Cochat P (2010) Genotype-phenotype correlation in primary hyperoxaluria type 1: the p.Gly170Arg AGXT mutation is associated with a better outcome. Kidney Int 77:443–449
Cochat P, Hulton SA, Acquaviva C, Danpure CJ, Daudon M, De Marchi M, Fargue S, Groothoff J, Harambat J, Hoppe B, Jamieson NV, Kemper MJ, Mandrile G, Marangella M, Picca S, Rumsby G, Salido E, Straub M, van Woerden CS (2012) Primary hyperoxaluria type 1: indications for screening and guidance for diagnosis and treatment. Nephrol Dial Transplant 27:1729–1736
Hoppe B, Kemper MJ, Bökenkamp A, Langman CB (1998) Plasma calcium-oxalate saturation in children with renal insufficiency and in children with primary hyperoxaluria. Kidney Int 54:921–925
Beck BB, Hoyer-Kuhn H, Göbel H, Habbig S, Hoppe B (2014) Hyperoxaluria and systemic oxalosis: an update on current therapy and future directions. Expert Opin Invest Drugs 22:117–129
Fargue S, Harambat J, Gagnadoux MF, Tsimaratos M, Janssen F, Llanas B, Berthélémé JP, Boudailliez B, Champion G, Guyot C, Macher MA, Nivet H, Ranchin B, Salomon R, Taque S, Rolland MO, Cochat P (2009) Effect of conservative treatment on the renal outcome of children with primary hyperoxaluria type 1. Kidney Int 76:767–773
Hoyer-Kuhn H, Kohbrok S, Volland R, Franklin J, Hero B, Beck BB, Hoppe B (2014) Vitamin B6 in primary hyperoxaluria I: first prospective trial after 40 years of practice. Clin J Am Soc Nephrol 9:468–477
Dawson KA, Allison MJ, Hartman PA (1980) Isolation and some characteristics of anaerobic oxalate degrading bacteria from the rumen. Appl Environ Microbiol 40:833–839
Allison MJ, Dawson KA, Mayberry WR, Foss JG (1985) Oxalobacter formigenes gen. nov. sp.nov.: oxalate-degrading anaerobes that inhabit the gastrointestinal tract. Arch Microbiol 141:1–7
Kelly JP, Curhan GC, Cave DR, Anderson TE, Kaufman DW (2011) Factors related to colonization with Oxalobacter formigenes in U.S. Adults. J Endourol 25:673–679
Knauf F, Lo N, Jiang Z, Robertson WG, van Italie CM, Anderson JM, Aronson PS (2011) Net intestinal transport of oxalate reflects passive absorption and SLC26A6-mediated secretion. J Am Soc Nephrol 22:2247–2255
Hatch M, Freel RW (2013) A human strain of Oxalobacter (HC-1) promotes enteric oxalate secretion in the small intestine of mice and reduces urinary oxalate excretion. Urolithiasis 41:379–384
Hoppe B, Beck B, Gatter N, von Unruh G, Tischer A, Hesse A, Laube N, Kaul P, Sidhu H (2006) Oxalobacter formigenes: a potential tool for the treatment of primary hyperoxaluria type 1. Kidney Int 70:1305–1311
Hoppe B, Groothoff JW, Hulton SA, Cochat P, Niaudet P, Kemper MJ, Deschênes G, Unwin R, Milliner D (2011) Efficacy and safety of Oxalobacter formigenes to reduce urinary oxalate in primary hyperoxaluria. Nephrol Dial Transplant 26:3609–3615
Wolthers BG, Hayer M (1982) The determination of oxalic acid in plasma and urine by means of capillary gas chromatography. Clin Chim Acta 120:87–102
Clifford-Mobley O, Sjögren A, Lindner E, Rumsby G (2016) Urine oxalate biological variation in patients with primary hyperoxaluria. Urolithiasis 44:333–337
Pascual E, Sivera F (2007) Time required for disappearance of urate crystals from synovial fluid after successful hypouricaemic treatment relates to the duration of gout. Ann Rheum Dis 66:1056–1058
Lagies R, Herberg U, Hoppe B, Feldkoetter M, Udink ten Cate F, Beck B (2015) Speckle Tracking Echocardiography detects impaired systolic function in patients with primary hyperoxaluria type I. Nieren und Hochdruckkrankheiten 44(2):74 (abstract 57)
Acknowledgements
The Department of Clinical Biochemistry at University College London Hospital, the Departments of Clinical Chemistry and Pediatrics at the Academic Medical Center, Amsterdam and the Institute for Microecology, Herborn were the central laboratories in this study. The investigators would also like to thank all of the patients who participated in this study. Medical writing services were paid for by OxThera and were provided by Drs James Serginson and Antigoni Ekonomou of Niche Science and Technology Limited.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The study was funded by OxThera Intellectual Property AB, Stockholm, Sweden.
This research has also received funding from the European Union’s Seventh Framework Programme managed by REA-Research Executive Agency http://ec.europa.eu/research/rea (FP7/2007–2013) under grant agreement no FP7-SME-2013.
Conflict of interest
Anna Sjögren and Elisabeth Lindner are employees at OxThera, the sponsor of the study. Bernd Hoppe has received consulting fees from OxThera.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online Resource 1
(DOCX 25 kb)
Online Resource 2
(DOCX 25 kb)
Online Resource 3
(DOCX 25 kb)
Online Resource 4
(DOCX 25 kb)
Online Resource 5
(DOCX 81 kb)
Online Resource 6
(DOCX 26 kb)
Online Resource 7
(DOCX 24 kb)
Rights and permissions
About this article
Cite this article
Hoppe, B., Niaudet, P., Salomon, R. et al. A randomised Phase I/II trial to evaluate the efficacy and safety of orally administered Oxalobacter formigenes to treat primary hyperoxaluria. Pediatr Nephrol 32, 781–790 (2017). https://doi.org/10.1007/s00467-016-3553-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-016-3553-8