Abstract
Background
Vesicoureteral reflux (VUR) is associated with an increased risk of kidney disorders. It is unclear whether VUR is associated with progression from chronic kidney disease (CKD) to end-stage kidney disease (ESKD) in children with congenital anomalies of the kidney and urinary tract (CAKUT).
Methods
We conducted a 3-year follow-up survey of a cohort of 447 children with CKD (stage 3–5). Rates of and risk factors for progression to ESKD were determined using the Kaplan–Meier method and Cox regression respectively.
Results
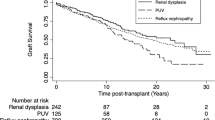
Congenital anomaly of the kidney and urinary tract was the primary etiology in 278 out of 447 children; 118 (42.4 %) had a history of VUR at the start of the cohort study. There were significantly more boys than girls with VUR, whereas the proportions were similar in children without VUR. The types of urinary anomalies/complications of the two groups were significantly different. Three-year renal survival rates of the groups were not significantly different, irrespective of CKD stage. Age < 2 years and age after puberty, stage 4 or 5 CKD, and heavy proteinuria, but not history of VUR, were significantly associated with progression to ESKD.
Conclusions
History of VUR at the start of follow-up was not associated with the progression of stage 3–5 CKD in children with CAKUT.

Similar content being viewed by others
References
Brandstrom P, Esbjorner E, Herthelius M, Swerkersson S, Jodal U, Hansson S (2010) The Swedish reflux trial in children: III. Urinary tract infection pattern. J Urol 184:286–291
Swerkersson S, Jodal U, Sixt R, Stokland E, Hansson S (2007) Relationship among vesicoureteral reflux, urinary tract infection and renal damage in children. J Urol 178:647–651
Salo J, Ikaheimo R, Tapiainen T, Uhari M (2011) Childhood urinary tract infections as a cause of chronic kidney disease. Pediatrics 128:840–847
Montini G, Tullus K, Hewitt I (2011) Febrile urinary tract infections in children. N Engl J Med 365:239–250
Shaikh N, Ewing AL, Bhatnagar S, Hoberman A (2010) Risk of renal scarring in children with a first urinary tract infection: a systematic review. Pediatrics 126:1084–1091
Chen MJ, Cheng HL, Chiou YY (2013) Risk factors for renal scarring and deterioration of renal function in primary vesico-ureteral reflux children: a long-term follow-up retrospective cohort study. PLoS One 8:e57954
Sjostrom S, Jodal U, Sixt R, Bachelard M, Sillen U (2009) Longitudinal development of renal damage and renal function in infants with high grade vesicoureteral reflux. J Urol 181:2277–2283
Coulthard MG (2009) Vesicoureteric reflux is not a benign condition. Pediatr Nephrol 24:227–232
Venhola M, Uhari M (2009) Vesicoureteral reflux, a benign condition. Pediatr Nephrol 24:223–226
Moorthy I, Easty M, McHugh K, Ridout D, Biassoni L, Gordon I (2005) The presence of vesicoureteric reflux does not identify a population at risk for renal scarring following a first urinary tract infection. Arch Dis Child 90:733–736
Hewitt IK, Zucchetta P, Rigon L, Maschio F, Molinari PP, Tomasi L, Toffolo A, Pavanello L, Crivellaro C, Bellato S, Montini G (2008) Early treatment of acute pyelonephritis in children fails to reduce renal scarring: data from the Italian Renal Infection Study Trials. Pediatrics 122:486–490
Mathews R, Carpenter M, Chesney R, Hoberman A, Keren R, Mattoo T, Moxey-Mims M, Nyberg L, Greenfield S (2009) Controversies in the management of vesicoureteral reflux: the rationale for the RIVUR study. J Pediatr Urol 5:336–341
Sung J, Skoog S (2012) Surgical management of vesicoureteral reflux in children. Pediatr Nephrol 27:551–561
Ishikura K, Uemura O, Ito S, Wada N, Hattori M, Ohashi Y, Hamasaki Y, Tanaka R, Nakanishi K, Kaneko T, Honda M, Pediatric CKD Study Group (2013) Pre-dialysis chronic kidney disease in children: results of a nationwide survey in Japan. Nephrol Dial Transplant 28:2345–2355
Ishikura K, Uemura O, Hamasaki Y, Ito S, Wada N, Hattori M, Ohashi Y, Tanaka R, Nakanishi K, Kaneko T, Honda M, Pediatric CKD Study Group (2014) Progression to end-stage kidney disease in Japanese children with chronic kidney disease: results of a nationwide prospective cohort study. Nephrol Dial Transplant 29:878–884
Matsuo N (1993) Skeletal and sexual maturation in Japanese children. Clin Pediatr Endocrinol 2 [Suppl 1]:1–4
Shaikh N, Hoberman A, Rockette HE, Kurs-Lasky M (2012) Identifying children with vesicoureteral reflux: a comparison of 2 approaches. J Urol 188:1895–1899
International Reflux Study Committee (1981) Medical versus surgical treatment of primary vesicoureteral reflux: report of the International Reflux Study Committee. Pediatrics 67:392–400
Uemura O, Honda M, Matsuyama T, Ishikura K, Hataya H, Yata N, Nagai T, Ikezumi Y, Fujita N, Ito S, Iijima K, Kitagawa T (2011) Age, gender, and body length effects on reference serum creatinine levels determined by an enzymatic method in Japanese children: a multicenter study. Clin Exp Nephrol 15:694–699
Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL (2009) New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20:629–637
Japanese Society of Nephrology (2014) Evidence-based clinical practice guideline for CKD 2013. Clin Exp Nephrol 18:346–423
Elder JS, Peters CA, Arant BS Jr, Ewalt DH, Hawtrey CE, Hurwitz RS, Parrott TS, Snyder HM 3rd, Weiss RA, Woolf SH, Hasselblad V (1997) Pediatric Vesicoureteral Reflux Guidelines Panel summary report on the management of primary vesicoureteral reflux in children. J Urol 157:1846–1851
Peters CA, Skoog SJ, Arant BS Jr, Copp HL, Elder JS, Hudson RG, Khoury AE, Lorenzo AJ, Pohl HG, Shapiro E, Snodgrass WT, Diaz M (2010) Summary of the AUA guideline on management of primary vesicoureteral reflux in children. J Urol 184:1134–1144
Silva JM, Diniz JS, Silva AC, Azevedo MV, Pimenta MR, Oliveira EA (2006) Predictive factors of chronic kidney disease in severe vesicoureteral reflux. Pediatr Nephrol 21:1285–1292
Acknowledgements
The authors would like to thank Drs Takuhito Nagai (Aichi), Kenichi Satomura (Osaka), Tomoo Kise (Okinawa), Takuji Yamada (Aichi), Midori Awazu (Tokyo), Hiroshi Asanuma (Tokyo), Toshiyuki Ohta (Hiroshima), Takeshi Matsuyama (Tokyo), Hidefumi Nakamura (Tokyo), Mayumi Sako (Tokyo), Tomoyuki Sakai (Shiga), Yusuke Okuda (Shiga), Shunsuke Shinozuka (Saitama), Yoshinobu Nagaoka (Hokkaido), Shuichiro Fujinaga (Saitama), Hiroshi Kitayama (Shizuoka), Naoya Fujita (Shizuoka), Masataka Hisano (Chiba), Daishi Hirano (Tokyo), Yuko Akioka (Tokyo), Naoaki Mikami (Tokyo), Hiroshi Hataya (Tokyo), Hiroyuki Satoh (Tokyo), Tae Omori (Tokyo), Takashi Sekine (Tokyo), Yoshimitsu Goto (Aichi), Yohei Ikezumi (Niigata), Takeshi Yamada (Niigata), and Akira Matsunaga (Yamagata) of The Pediatric CKD Study Group in Japan for their contributions to the study. The authors would also like to thank all the institutions that participated in the surveys listed in the Supplement, and Mr Masaaki Kurihara, Ms Chie Matsuda, Ms Naomi Miyamoto, and Ms Takako Arai of the Japan Clinical Research Support Unit (Tokyo) for their help with data management; Dr Naoaki Mikami and Ms Sachiko Kawabe of Tokyo Metropolitan Children’s Medical Center for their contribution to manuscript preparation; and Nicholas Smith, PhD, of Edanz Group Ltd., for providing language editorial support in the preparation of the manuscript. The results presented in this paper have not been published previously in whole or part, except in abstract format.
Ethics
The study was conducted in accordance with the principles of the Declaration of Helsinki and the ethical guidelines issued by the Ministry of Health, Labour, and Welfare, Japan. The study was approved by the ethics committee of the Tokyo Metropolitan Children’s Medical Center (ID: 23–49). Because data were reported using patient medical records, informed consent was not obtained in accordance with the above guidelines.
Funding
This work was supported by a Health and Labour Sciences Research Grant for Research on Rare and Intractable Diseases from the Ministry of Health, Labour, and Welfare, Japan (H25-nanchitou(nan)-ippan-017 and H26-nanchitou(nan)-ippan-036) and the 2013 Tokyo Metropolitan Hospitals’ Clinical Research Fund (Special Research).
Conflict of interest
Kenji Ishikura has received lecture fees from Novartis Pharma and Asahi Kasei Pharma. Osamu Uemura has received lecture fees from Asahi Kasei Pharma, Kyowa Hakko Kirin, Takeda Pharmaceutical, and Siemens Group in Japan. Yuko Hamasaki has received research grants from Novartis Pharma, and lecture fees from Novartis Pharma, Astellas Pharma, and Pfizer Japan. Hideo Nakai has received a research grant from Astellas Pharma. Ryojiro Yasuo Ohashi has received research grants from Kyowa Hakko Kirin and Chugai pharmaceutical. Tanaka has received lecture fees from Pfizer Japan and Asahi Kasei Pharma. Koichi Nakanishi has received lecture fees from Novartis Pharma, Asahi Kasei Pharma, and Astellas Pharma. Kazumoto Iijima has received research grants from Novartis and Pfizer Japan, and lecture fees from Novartis, Asahi Kasei Pharma, and Pfizer Japan. Masataka Honda has received lecture fees from Novartis Pharma, Asahi Kasei Pharma, Takeda Pharmaceutical, and Chugai Pharmaceutical. Drs Ito, Harada, Hattori, and Mr Kaneko have no conflicts of interest to declare.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Ishikura, K., Uemura, O., Hamasaki, Y. et al. Insignificant impact of VUR on the progression of CKD in children with CAKUT. Pediatr Nephrol 31, 105–112 (2016). https://doi.org/10.1007/s00467-015-3196-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-015-3196-1