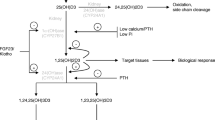
Abstract
Severe vitamin D deficiency (reduction in serum 25(OH)D concentration) in infants and children can cause features of the Fanconi syndrome, including phosphaturia, glycosuria, aminoaciduria, and renal tubular acidosis. This indicates that vitamin D and its metabolites influence proximal tubule function. Filtered 25(OH)D bound to vitamin D binding protein (DBP) is endocytosed by megalin-cubilin in the apical membrane. Intracellular 25(OH)D is metabolized to 1,25(OH)2D or calcitroic acid by 1-α-hydroxylase or 24-hydroxylase in tubule cell mitochondria. Bone-produced fibroblast growth factor 23 (FGF23) bound to Klotho in tubule cells and intracellular phosphate concentrations are regulators of 1-α-hydroxylase activity and cause proximal tubule phosphaturia. Aminoaciduria occurs when amino acid transporter synthesis is deficient, and 1,25(OH)2D along with retinoic acid up-regulate transporter synthesis by a vitamin D response element in the promoter region of the transporter gene. This review discusses evidence gained from studies in animals or cell lines, as well as from human disorders, that provide insight into vitamin D–proximal tubule interactions.



Similar content being viewed by others
References
Chesney RW, Harrison HE (1975) Fanconi syndrome following bowel surgery and hepatitis reversed by 25-hydroxycholecalciferol. J Pediatr 86:857–861
Guignard JP, Torrado A (1973) Proximal renal tubular acidosis in vitamin D deficiency rickets. Acta Paediatr Scand 62:543–546
Curthoys NP, Moe OW (2014) Proximal tubule function and response to acidosis. Clin J Am Soc Nephrol 9:1627–1638
Dusso AS, Brown AJ, Slatopolsky E (2005) Vitamin D. Am J Physiol Renal Physiol 289:F8–F28
Holick MF (2007) Vitamin D deficiency. N Engl J Med 357:266–281
Christensen EI, Willnow TE (1999) Essential role of megalin in renal proximal tubule for vitamin homeostasis. J Am Soc Nephrol 10:2224–2236
Negri AL (2006) Proximal tubule endocytic apparatus as the specific renal uptake mechanism for vitamin D-binding protein/25-(OH)D3 complex. Nephrology (Carlton) 11:510–515
Kaseda R, Hosojima M, Sato H, Saito A (2011) Role of megalin and cubilin in the metabolism of vitamin D(3). Ther Apher Dial 15(Suppl 1):14–17
Nykjaer A, Fyfe JC, Kozyraki R, Leheste JR, Jacobsen C, Nielsen MS, Verroust PJ, Aminoff M, de la Chapelle A, Moestrup SK, Ray R, Gliemann J, Willnow TE, Christensen EI (2001) Cubilin dysfunction causes abnormal metabolism of the steroid hormone 25(OH) vitamin D(3). Proc Natl Acad Sci U S A 98:13895–13900
Gräsbeck R (2006) Imerslund-Gräsbeck syndrome (selective vitamin B(12) malabsorption with proteinuria). Orphanet J Rare Dis 1:17
Storm T, Zeitz C, Cases O, Amsellem S, Verroust PJ, Madsen M, Benoist JF, Passemard S, Lebon S, Jonsson IM, Emma F, Koldso H, Hertz JM, Nielsen R, Christensen EI, Kozyraki R (2013) Detailed investigations of proximal tubular function in Imerslund-Gräsbeck syndrome. BMC Med Genet 14:111
Chlon TM, Taffany DA, Welsh J, Rowling MJ (2008) Retinoids modulate expression of the endocytic partners megalin, cubilin, and disabled-2 and uptake of vitamin D-binding protein in human mammary cells. J Nutr 138:1323–1328
Rowling MJ, Kemmis CM, Taffany DA, Welsh J (2006) Megalin-mediated endocytosis of vitamin D binding protein correlates with 25-hydroxycholecalciferol actions in human mammary cells. J Nutr 136:2754–2759
Hagenfeldt Y, Berlin T (1992) The human renal 25-hydroxyvitamin D3-1 alpha-hydroxylase: properties studied by isotope-dilution mass spectrometry. Eur J Clin Invest 22:223–228
Yoshida T, Yoshino J, Hayashi M, Saruta T (2002) Identification of a renal proximal tubular cell-specific enhancer in the mouse 25-hydroxyvitamin d 1alpha-hydroxylase gene. J Am Soc Nephrol 13:1455–1463
Perwad F, Portale AA (2011) Vitamin D metabolism in the kidney: regulation by phosphorus and fibroblast growth factor 23. Mol Cell Endocrinol 347:17–24
Weber TJ, Liu S, Indridason OS, Quarles LD (2003) Serum FGF23 levels in normal and disordered phosphorus homeostasis. J Bone Miner Res 18:1227–1234
Monkawa T, Yoshida T, Wakino S, Shinki T, Anazawa H, Deluca HF, Suda T, Hayashi M, Saruta T (1997) Molecular cloning of cDNA and genomic DNA for human 25-hydroxyvitamin D3 1 alpha-hydroxylase. Biochem Biophys Res Commun 239:527–533
St-Arnaud R, Messerlian S, Moir JM, Omdahl JL, Glorieux FH (1997) The 25-hydroxyvitamin D 1-alpha-hydroxylase gene maps to the pseudovitamin D-deficiency rickets (PDDR) disease locus. J Bone Miner Res 12:1552–1559
Ranch D, Zhang MY, Portale AA, Perwad F (2011) Fibroblast growth factor 23 regulates renal 1,25-dihydroxyvitamin D and phosphate metabolism via the MAP kinase signaling pathway in Hyp mice. J Bone Miner Res 26:1883–1890
Andrukhova O, Zeitz U, Goetz R, Mohammadi M, Lanske B, Erben RG (2012) FGF23 acts directly on renal proximal tubules to induce phosphaturia through activation of the ERK1/2-SGK1 signaling pathway. Bone 51:621–628
Lindberg K, Amin R, Moe OW, Hu MC, Erben RG, Ostman Wernerson A, Lanske B, Olauson H, Larsson TE (2014) The kidney is the principal organ mediating Klotho effects. J Am Soc Nephrol 25:2169–2175
Martin A, Quarles LD (2012) Evidence for FGF23 involvement in a bone-kidney axis regulating bone mineralization and systemic phosphate and vitamin D homeostasis. Adv Exp Med Biol 728:65–83
Chanakul A, Zhang MY, Louw A, Armbrecht HJ, Miller WL, Portale AA, Perwad F (2013) FGF-23 regulates CYP27B1 transcription in the kidney and in extra-renal tissues. PLoS One 8:e72816
Matsumoto T, Ikeda K, Morita K, Fukumoto S, Takahashi H, Ogata E (1987) Blood Ca2+ modulates responsiveness of renal 25(OH)D3-1 alpha-hydroxylase to PTH in rats. Am J Physiol 253:E503–E507
Maiti A, Hait NC, Beckman MJ (2008) Extracellular calcium-sensing receptor activation induces vitamin D receptor levels in proximal kidney HK-2G cells by a mechanism that requires phosphorylation of p38alpha MAPK. J Biol Chem 283:175–183
Fanconi G (1951) Chronic disorders of calcium and phosphate metabolism in children. Schweiz Med Wochenschr 81:908–913
Lightwood R, Stapleton T (1953) Idiopathic hypercalcaemia in infants. Lancet 265:255–256
Schlingmann KP, Kaufmann M, Weber S, Irwin A, Goos C, John U, Misselwitz J, Klaus G, Kuwertz-Broking E, Fehrenbach H, Wingen AM, Guran T, Hoenderop JG, Bindels RJ, Prosser DE, Jones G, Konrad M (2011) Mutations in CYP24A1 and idiopathic infantile hypercalcemia. N Engl J Med 365:410–421
Chen KS, Prahl JM, DeLuca HF (1993) Isolation and expression of human 1,25-dihydroxyvitamin D3 24-hydroxylase cDNA. Proc Natl Acad Sci U S A 90:4543–4547
Dowen FE, Sayers JA, Hynes AM, Sayer JA (2014) CYP24A1 mutation leading to nephrocalcinosis. Kidney Int 85:1475
Meusburger E, Mundlein A, Zitt E, Obermayer-Pietsch B, Kotzot D, Lhotta K (2013) Medullary nephrocalcinosis in an adult patient with idiopathic infantile hypercalcaemia and a novel CYP24A1 mutation. Clin Kidney J 6:211–215
Dinour D, Davidovits M, Aviner S, Ganon L, Michael L, Modan-Moses D, Vered I, Bibi H, Frishberg Y, Holtzman EJ (2015) Maternal and infantile hypercalcemia caused by vitamin-D-hydroxylase mutations and vitamin D intake. Pediatr Nephrol 30:145–152
Kantarci S, Al-Gazali L, Hill RS, Donnai D, Black GC, Bieth E, Chassaing N, Lacombe D, Devriendt K, Teebi A, Loscertales M, Robson C, Liu T, MacLaughlin DT, Noonan KM, Russell MK, Walsh CA, Donahoe PK, Pober BR (2007) Mutations in LRP2, which encodes the multiligand receptor megalin, cause Donnai–Barrow and facio-oculo-acoustico-renal syndromes. Nat Genet 39:957–959
Genetics Home Reference. Donnai–Barrow syndrome. (2014) http://ghr.nlm.nih.gov/condition/Donnai–Barrow-syndrome. Accessed 12/12/14
Wahlstedt-Froberg V, Pettersson T, Aminoff M, Dugue B, Gräsbeck R (2003) Proteinuria in cubilin-deficient patients with selective vitamin B12 malabsorption. Pediatr Nephrol 18:417–421
Orphanet: Proximal renal tubular acidosis. (2014) http://www.orpha.net/consor4.01/www/cgi-bin/OC_Exp.php?lng=EN&Expert=47159 Accessed 10/6/14
Chisolm JJ Jr (1959) The clinical significance of aminoaciduria. J Pediatr 55:303–314
Hassanein EA, Patel H (1967) The parathyroid hormone and aminoaciduria in vitamin-D deficiency rickets. Acta Paediatr Scand 56:445–449
Jonxis JH, Huisman TH (1953) Amino-aciduria in rachitic children. Lancet 265:428–431
Fraser D, Kooh SW, Scriver CR (1967) Hyperparathyroidism as the cause of hyperaminoaciduria and phosphaturia in human vitamin D deficiency. Pediatr Res 1:425–435
Harrison HE (1959) Physiology of vitamin D. Helv Paediatr Acta 14:434–446
Scriver CR (1974) Rickets and the pathogenesis of impaired tubular transport of phosphate and other solutes. Am J Med 57:43–49
Grose JH, Scriver CR (1968) Parathyroid-dependent phosphaturia and aminoaciduria in the vitamin D-deficient rat. Am J Physiol 214:370–377
Phillips ME, Havard J, Otterud B (1980) Aminoaciduria in chronic renal failure–its relationship to vitamin D and parathyroid status. Am J Clin Nutr 33:1541–1545
Drezner MK, Feinglos MN (1977) Osteomalacia due to 1alpha,25-dihydroxycholecalciferol deficiency. association with a giant cell tumor of bone. J Clin Invest 60:1046–1053
Cusworth DC, Dent CE, Scriver CR (1972) Primary hyperparathyroidism and hyperaminoaciduria. Clin Chim Acta 41:355–361
Brodehl J, Kaas WP, Weber HP (1971) Clearance of free amino acids in vitamin D deficiency rickets before and following therapy with vitamin D. Monatsschr Kinderheilkd 119:401–406
Weber HP, Brodehl J, Jakel A (1973) Influence of phosphate load on renal amino-acid transport. Monatsschr Kinderheilkd 121:324–326
Phillips ME (1980) Aminoaciduria–its relationship to vitamin D and parathyroid hormone. Crit Rev Clin Lab Sci 12:215–239
Dabbagh S, Chesney R, Gusowski N, Mathews MC, Padilla M, Theissen M, Slatopolsky E (1989) Aminoaciduria of vitamin D deficiency is independent of PTH levels and urinary cyclic AMP. Miner Electrolyte Metab 15:221–232
Dabbagh S, Gusowski N, Chesney R, Falsetti G, Ellis M, Ellis D (1989) Cyclic AMP does not alter taurine accumulation by rat renal brush border membrane vesicles. Biochem Med Metab Biol 42:132–145
Dabbagh S, Gusowski N, Padilla M, Theissen M, Chesney RW (1990) Perturbation of renal amino acid transport by brush border membrane vesicles in the vitamin D-deficient rat. Biochem Med Metab Biol 44:64–76
Shafeghati Y, Momenin N, Esfahani T, Reyniers E, Wuyts W (2008) Vitamin D-dependent rickets type II: report of a novel mutation in the vitamin D receptor gene. Arch Iran Med 11:330–334
Chesney RW, Han X (2013) Differential regulation of TauT by calcitriol and retinoic acid via VDR/RXR in LLC-PK1 and MCF-7 cells. Adv Exp Med Biol 776:291–305
Carlberg C, Seuter S (2009) A genomic perspective on vitamin D signaling. Anticancer Res 29:3485–3493
Kawashima H, Kraut JA, Kurokawa K (1982) Metabolic acidosis suppresses 25-hydroxyvitamin in D3-1alpha-hydroxylase in the rat kidney. distinct site and mechanism of action. J Clin Invest 70:135–140
Kraut JA, Gordon EM, Ransom JC, Horst R, Slatopolsky E, Coburn JW, Kurokawa K (1983) Effect of chronic metabolic acidosis on vitamin D metabolism in humans. Kidney Int 24:644–648
Haque SK, Ariceta G, Batlle D (2012) Proximal renal tubular acidosis: a not so rare disorder of multiple etiologies. Nephrol Dial Transplant 27:4273–4287
Alper SL (2010) Familial renal tubular acidosis. J Nephrol 23(Suppl 16):S57–S76
Acknowledgments
I would like to thank Shermine Dabbaugh, Xiaobin Han, and Andrea B. Patters for their contributions to this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chesney, R.W. Interactions of vitamin D and the proximal tubule. Pediatr Nephrol 31, 7–14 (2016). https://doi.org/10.1007/s00467-015-3050-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-015-3050-5