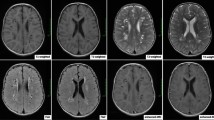
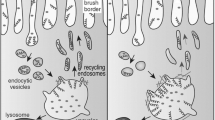
Abstract
Background
The oculocerebrorenal syndrome of Lowe (OCRL) is a rare X-linked multi-systemic disorder, almost always characterized by the triad of congenital cataract, cognitive and behavioral impairment and a proximal tubulopathy.
Methods
Twenty-eight novel patients with suspected Lowe syndrome were studied.
Results
All patients carried OCRL gene defects with mutational hot spots at CpG dinucleotides. Mutations previously unknown in Lowe syndrome were observed in ten of the 28 patients, and carriership was identified in 30.4 % of the mothers investigated. Mapping the exact breakpoints of a complete OCRL gene deletion revealed involvement of several flanking repeat elements. We noted a similar pattern of documented clinically relevant symptoms, and even though the patient cohort comprised relatively young patients, 32 % of these patients already showed advanced chronic kidney disease. Thrombocytopenia was seen in several patients, and hyperosmia and/or hyperacusis were reported recurrently. A p.Asp523Asn mutation in a Polish patient, associated with the typical cerebrorenal spectrum but with late cataract (10 year), was also evident in two milder affected Italian brothers with ocular involvement of similar progression.
Conclusions
We have identified clinical features in 28 patients with suspected Lowe syndrome that had not been recognized in Lowe syndrome prior to our study. We also provide further evidence that OCRL mutations cause a phenotypic continuum with selective and/or time-dependent organ involvement. At least some of these mutants might exhibit a genotype–phenotype correlation.


Similar content being viewed by others
References
Lowe CU, Terrey M, MacLachlan EA (1952) Organic-aciduria, decreased renal ammonia production, hydrophthalmos, and mental retardation; a clinical entity. AMA Am J Dis Child 83:164–184
Loi M (2006) Lowe syndrome. Orphanet J Rare Dis 1:16
Böckenhauer D, Bokenkamp A, van’t Hoff W, Levtchenko E, Kist-van Holthe JE, Tasic V, Ludwig M (2008) Renal phenotype in Lowe syndrome: a selective proximal tubular dysfunction. Clin J Am Soc Nephrol 3:1430–1436
Kleta R (2008) Fanconi or not Fanconi? Lowe syndrome revisited. Clin J Am Soc Nephrol 3:1244–1245
Laube GF, Russell-Eggitt IM, van’t Hoff WG (2004) Early proximal tubular dysfunction in Lowe’s syndrome. Arch Dis Child 89:479–480
Charnas L, Bernar J, Pereshkpour GH, Dalakas M, Harper GS, Gahal WA (1988) MRI findings and peripheral neuropathy in Lowe syndrome. Neuropediatrics 19:7–9
Demmer LA, Wippold FJ 2nd, Dowton SB (1992) Periventricular white matter cystic lesions in Lowe (oculocerebrorenal) syndrome: a new MRI finding. Pediatr Radiol 22:76–77
Lasne D, Baujat G, Mirault T, Lunardi J, Grelac F, Egot M, Salomon R, Bachelot-Loza C (2010) Bleeding disorders in Lowe syndrome patients: evidence for a link between OCRL mutations and primary haemostasis disorders. Br J Haematol 150:685–688
McSpadden K (2010) Living with Lowe syndrome: a guide for families, friends and professionals, 4th edn. Lowe Syndrome Association, Inc, Plano
Nussbaum RL, Orrison BM, Jänne PA, Charnas L, Chinault AC (1997) Physical mapping and genomic structure of the Lowe syndrome gene OCRL1. Hum Genet 99:145–150
Hichri H, Rendu J, Monnier N, Coutton C, Dorseuil O, Vargas Poussou R, Beaujat G, Blanchard A, Nobili F, Ranchin B, Remesey M, Salomon R, Satre V, Lunardi J (2011) From Lowe syndrome to Dent disease: correlations between mutations of the OCRL1 gene and clinical and biochemical phenotypes. Hum Mutat 32:379–388
Hoopes RR Jr, Shrimpton AE, Knohl SJ, Hueber P, Hoppe B, Matyus J, Simckes A, Tasic V, Toenshoff B, Suchy SF, Nussbaum RL, Scheinman SJ (2005) Dent disease with mutation in OCRL1. Am J Hum Genet 76:260–267
Utsch B, Bökenkamp A, Benz MR, Besbas N, Dötsch J, Franke I, Fründ S, Gok F, Hoppe B, Karle S, Kuwertz-Bröking E, Laube G, Neb M, Nuutinen M, Ozaltin F, Rascher W, Ring T, Tasic V, van Wijk JA, Ludwig M (2006) Novel OCRL1 mutations in patients with the phenotype of Dent disease. Am J Kidney Dis 48:942–954
Böckenhauer D, Bökenkamp A, Nuutinen M, Unwin R, van’t Hoff W, Sirimanna T, Vrljicak K, Ludwig M (2012) Novel OCRL mutations in patients with Dent-2 disease. J Pediatr Genet 1:15–23
Bökenkamp A, Böckenhauer D, Cheong HI, Hoppe B, Tasic V, Unwin R, Ludwig M (2009) Dent-2 disease: a mild variant of Lowe syndrome. J Pediatr 155:94–99
Recker F, Reutter H, Ludwig M (2013) Lowe syndrome/Dent-2 disease: a comprehensive review of known and novel aspects. J Pediatr Genet 2:53–68
Mehta ZB, Pietka G, Lowe M (2014) The cellular and physiological functions of the Lowe syndrome protein OCRL1. Traffic 15:471–487
Ghazali S, Barratt TM (1974) Urinary excretion of calcium and magnesium in children. Arch Intern Med 49:97–101
Kruse K, Kracht U, Gopfert G (1982) Renal threshold phosphate concentration (TmPO4/GFR). Arch Intern Med 57:217–223
Schwartz GJ, Brion LP, Spitzer A (1987) The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin N Am 4:571–590
Tosetto E, Addis M, Caridi G, Meloni C, Emma F, Vergine G, Stringini G, Papalia T, Barbano G, Ghiggeri GM, Ruggeri L, Miglietti N, D’Angelo A, Melis MA, Anglani F (2009) Locus heterogeneity of Dent’s disease: OCRL1 and TMEM27 genes in patients with no CLCN5 mutations. Pediatr Nephrol 24:1967–1973
Draaken M, Giesen CA, Kesselheim AL, Jabs R, Aretz S, Kugaudo M, Chrzanowska KH, Krajewska-Walasek M, Ludwig M (2011) Maternal de novo triple mosaicism for two single OCRL nucleotide substitutions (c.1736A>T, c.1736A>G) in a Lowe syndrome family. Hum Genet 129:513–519
Peverall J, Edkins E, Goldblatt J, Murch A (2000) Identification of a novel deletion of the entire OCRL1 gene detected by FISH analysis in a family with Lowe syndrome. Clin Genet 58:479–482
Addis M, Meloni C, Congiu R, Santaniello S, Emma F, Zuffardi O, Cao A, Ciccone R, Melis MA, Cau M (2007) A novel interstitial deletion in Xq25, identified by array-CGH in a patient with Lowe syndrome. Eur J Med Genet 50:79–84
Mahmoudi H, Tug E, Parlak AH, Atasoy HI, Ludwig M, Polat M, Pasternack SM, Betz RC (2012) Identification of an Alu-mediated 12.2-kb deletion of the complete LPAR6 (P2RY5) gene in a Turkish family with hypotrichosis and wolly hair. Exp Dermatol 21:469–471
Keilhauer CN, Gal A, Sold JE, Zimmermann J, Netzer KO, Schramm L (2007) Clinical findings in a patient with Lowe syndrome and a splice site mutation in the OCRL1 gene. Klin Monatsbl Augenheilkd 224:207–209
Pasternack SM, Böckenhauer D, Refke M, Tasic V, Draaken M, Conrad C, Betz RC, Born M, Reutter H, Ludwig M (2013) A premature termination mutation in a patient with Lowe syndrome without congenital cataracts: dropping the “O” in OCRL. Klin Padiatr 225:29–33
Cho HY, Lee BH, Choi HJ, Ha IS, Choi Y, Cheong HI (2008) Renal manifestations of Dent disease and Lowe syndrome. Pediatr Nephrol 23:243–249
Kim HK, Kim JH, Kim YM, Kim GH, Lee BH, Choi JH, Yoo HW (2014) Lowe syndrome: a single center’s experience in Korea. Korean J Pediatr 57:140–148
Matzaris M, Jackson SP, Laxminarayan KM, Speed CJ, Mitchell CA (1994) Identification and characterization of the phosphatidylinositol-(4,5)-bisphosphate 5-phosphatase in human platelets. J Biol Chem 269:3397–3402
Taher AT, Arabi M, Sibai H, Nasreddine W, Otrock ZK, Musallam KM, Beydoun A (2012) Carbamazepine-induced thrombocytopenia. Blood Cells Mol Dis 48:197–198
Verrotti A, Scaparotta A, Grosso S, Chiarelli F, Coppola G (2014) Anticonvulsant drugs and hematological disease. Neurol Sci 35:983–993
Forge A, Wright T (2002) The molecular architecture of the inner ear. Br Med Bull 63:5–24
Cao H, Yin X, Cao Y, Jin Y, Wang S, Kong Y, Chen Y, Gao J, Heller S, Xu Z (2013) FCHSD1 and FCHSD2 are expressed in hair cell stereocilia and cuticular plate and regulate actin polymerization in vitro. PLoS ONE 8:e56516
Nández R, Balkin DM, Messa M, Liang L, Paradise S, Czapla H, Hein MY, Duncan JS, Mann M, De Camilli P (2014) A role of OCRL in clathrin-coated pit dynamics and uncoating revealed by studies of Lowe syndrome cells. Elife 3:e02975. doi: 10.7554/eLife.02975
Coon BG, Hernandez V, Madhivanan K, Mukherjee D, Hanna CB, Barinaga-Rementeria RI, Lowe M, Beales PL, Aguilar RC (2012) The Lowe syndrome protein OCRL1 is involved in primary cilia assembly. Hum Mol Genet 21:1835–1847
Luo N, West CC, Murga-Zamalloa CA, Sun L, Anderson RM, Wells CD, Weinreb RN, Travers JB, Khanna H, Sun Y (2012) OCRL localizes to the primary cilium: a new role for cilia in Lowe syndrome. Hum Mol Genet 21:3333–3344
Sharma N, Berbari NF, Yoder BK (2008) Ciliary dysfunction in the developmental abnormalities and diseases. Curr Top Dev Biol 85:371–427
Sekine T, Nozu K, Iyengar R, Fu XJ, Matsuo M, Tanaka R, Iijima K, Matsui E, Harita Y, Inatomi J, Igarashi T (2007) OCRL1 mutations in patients with Dent disease phenotype in Japan. Pediatr Nephrol 22:975–980
Shrimpton AE, Hoopes RR Jr, Knohl SJ, Hueber P, Reed AAC, Christie PT, Igarashi T, Lee P, Lehman A, White C, Milford DV, Sanchez MR, Unwin R, Wrong OM, Thakker RV, Scheinman SJ (2009) OCRL1 mutations in Dent 2 patients suggest a mechanism for phenotypic variability. Nephron Physiol 112:27–36
Skuta GL, Sugar J, Ericson ES (1983) Corneal endothelial cell measurements in megalocornea. Arch Ophthalmol 101:51–53
Webb TR, Matarin M, Gardner JC, Kelberman D, Hassan H, Ang W, Michaelides M, Ruddle JB, Pennell CE, Yazar S, Khor CC, Aung T, Yogarajah M, Robson AG, Holder GE, Cheetham ME, Traboulsi EI, Moore AT, Sowden JC, Sisodiya SM, Mackey DA, Tuft SJ, Hardcastle AJ (2012) X-linked megalocornea caused by mutations in CHRDL1 identifies an essential role for venotropin in anterior segment development. Am J Hum Genet 90:247–259
Hanefeld FA (1999) Oligogyric microcephaly. Neuropediatrics 30:102–103
Pang T, Atefy R, Sheen V (2008) Malformations of cortical development. Neurologist 14:181–191
Gropman A, Levin S, Yao L, Lin T, Suchy S, Sabnis S, Hadley D, Nussbaum R (2000) Unusual renal features of Lowe syndrome in a mildly affected boy. Am J Med Genet 95:461–466
Marques A, Ramos L, Gomes C, Correia AJ (2010) Lowe syndrome. Case report of a patient with a missense mutation in the OCRL1 gene. Port J Nephrol Hypertens 24:239–242
Youssoufian H, Kazazian HH Jr, Phillips DG, Aronis S, Tsiftis G, Brown VA, Antonarakis SE (1986) Recurrent mutations in haemophilia A give evidence for CpG mutation hotspots. Nature 324:380–382
Cooper DN, Krawczak M (1993) Human gene mutation. BIOS Scientic Publishers Limited, Oxford, pp 109–127
Ten Kate LP (1984) The significance of new mutations for the genetic epidemiology of Duchenne muscular dystrophy. In: Ten Kate LP, Pearson PL, Stadhouders AM (eds) Research into the origin and treatment of muscular dystrophy. Excerpta Medica, Amsterdam, pp 3–6
Acknowledgments
We are grateful to the patients and their parents for their invaluable contributions. We thank Dr. Andrzej Blumczyński for his help with patient recruitment. We would also like to thank Hartmut Engels for performing array analysis and Pia Uerdingen and Markus Draaken for excellent technical assistance. Funding for this study was provided by the European Union, FP7 (grant agreement 2012-305608 “European Consortium for High-Throughput Research in Rare Kidney Diseases (EURenOmics)” to DB.
Author information
Authors and Affiliations
Corresponding author
Additional information
Florian Recker and Marcin Zaniew contributed equally to this work.
Rights and permissions
About this article
Cite this article
Recker, F., Zaniew, M., Böckenhauer, D. et al. Characterization of 28 novel patients expands the mutational and phenotypic spectrum of Lowe syndrome. Pediatr Nephrol 30, 931–943 (2015). https://doi.org/10.1007/s00467-014-3013-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-014-3013-2