Abstract
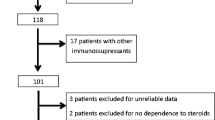
Mycophenolate mofetil (MMF) has emerged as a new therapeutic option in steroid-dependent nephrotic syndrome (SDNS). We conducted a phase II Bayesian trial of MMF in children with SDNS. Phase II trials, usually single-arm studies, investigate the effect of new treatments. Standard Fleming’s procedure relies on observed results (relapse rate during the trial), whereas Bayesian approach combines observed results with prior information (expected relapse rate according to prior studies and clinical experience). All patients were required to have received prior alkylating-agent treatment. Sixty-seven percent of them had also received levamisole. Patients received MMF (1,200 mg/m2/day) and prednisone according to a defined schedule [reduction of alternate-day (e.o.d) dose to 50% of pre-MMF dose at 3 months, 25% at 6 months]. Twenty-four children (median age 6.0 years, 2.8–14.4) entered the study and 23 completed it. Bayesian analysis showed that adding four patients would not change significance of results, allowing stopping inclusions. Four patients relapsed during the first 6 months (estimated probability 17.6%, 95% credibility interval: 5.4–35.0%) and two at months 8 and 11.5. In the 19 patients free of relapse during the first 6 months, median (Q1–Q3) prednisone maintenance dose decreased from 25 (10–44) to 9 (7.5–11.2) mg/m2 e.o.d (p < 0.001) and cumulative dose from 459 (382–689) to 264 (196–306) mg/m2/month (p < 0.001) before and on MMF respectively. Pre-MMF patient characteristics and MMF pharmacokinetics did not differ between patients with or without relapse. MMF reduces relapse rate and steroid dose in children with SDNS and should be proposed before cyclosporine and cyclophosphamide.
Similar content being viewed by others
References
Eddy AA, Symons JM (2003) Nephrotic syndrome in childhood. Lancet 362:629–623
Latta K, von Schnakenburg C, Ehrich JH (2001) A meta-analysis of cytotoxic treatment for frequently relapsing nephrotic syndrome in children. Pediatr Nephrol 16:271–282
Hodson EM, Willis NS, Craig JC (2008) Non-corticosteroid treatment for nephrotic syndrome in children. Cochrane Database Syst Revue.(1):CD002290
Cattran DC, Alexopoulos E, Heering P, Hoyer PF, Johnston A, Meyrier A, Ponticelli C, Saito T, Choukroun G, Nachman P, Praga (2007) Cyclosporin in idiopathic glomerular disease associated with the nephrotic syndrome: workshop recommendations. Kidney Int 72:1429–1447
Kengne-Wafo S, Massella L, Diomedi-Camassei F, Gianviti A, Vivarelli M, Greco M, Stringini GR, Emma F (2009) Risk factors for cyclosporin A nephrotoxicity in children with steroid-dependant nephrotic syndrome. Clin J Am Soc Nephrol 4:1409–1416
Barletta GM, Smoyer WE, Bunchman TE, Flynn JT, Kershaw DB (2003) Use of mycophenolate mofetil in steroid-dependent and -resistant nephrotic syndrome. Pediatr Nephrol 18:833–837
Gellermann J, Querfeld U (2004) Frequently relapsing nephrotic syndrome: treatment with mycophenolate mofetil. Pediatr Nephrol 19:101–104
Ulinski T, Dubourg L, Saïd MH, Parchoux B, Ranchin B, Cochat P (2005) Switch from cyclosporine A to mycophenolate mofetil in nephrotic children. Pediatr Nephrol 20:482–485
Novak I, Frank R, Vento S, Vergara M, Gauthier B, Trachtman H (2005) Efficacy of mycophenolate mofetil in pediatric patients with steroid-dependent nephrotic syndrome. Pediatr Nephrol 20:1265–126
Al-Akash S, Al-Makdama A (2005) Mycophenolate mofetil in children with steroid-dependent and/or frequently relapsing nephrotic syndrome. Ann Saudi Med 25:380–384
Frank C, Herrmann M, Fernandez S, Dirnecker D, Böswald M, Kolowos W, Ruder H, Haas JP (2000) Dominant T cells in idiopathic nephrotic syndrome of childhood. Kidney Int 57:510–517
van den Berg JG, Weening JJ (2004) Role of the immune system in the pathogenesis of idiopathic nephrotic syndrome. Clin Sci Lond 107:125–136
Zhang S, Audard V, Fan Q, Pawlak A, Lang P, Sahali D (2011) Immunopathogenesis of idiopathic nephrotic syndrome. Contrib Nephrol 169:94–106
Bagga A, Hari P, Moudgil A, Jordan SC (2003) Mycophenolate mofetil and prednisolone therapy in children with steroid-dependent nephrotic syndrome. Am J Kidney Dis 4:1114–1120
Mendizábal S, Zamora I, Berbel O, Sanahuja MJ, Fuentes J, Simon J (2005) Mycophenolate mofetil in steroid/cyclosporine-dependent/resistant nephrotic syndrome. Pediatr Nephrol 20:914–919
Hogg RJ, Fitzgibbons L, Bruick J, Bunke M, Ault B, Baqi N, Trachtman H, Swinford R (2006) Mycophenolate mofetil in children with frequently relapsing nephrotic syndrome: a report from the Southwest Pediatric Nephrology Study Group. Clin J Am Soc Nephrol 1:1173–1178
Fujinaga S, Ohtomo Y, Umino D, Takemoto M, Shimizu T, Yamashiro Y, Kaneko K (2007) A prospective study on the use of mycophenolate mofetil in children with cyclosporine-dependent nephrotic syndrome. Pediatr Nephrol 22:71–76
Dorresteijn EM, Kist-van Holthe JE, Levtchenko EN, Nauta J, Hop WC, van der Heijden AJ (2008) Mycophenolate mofetil versus cyclosporine for remission maintenance in nephrotic syndrome. Pediatr Nephrol 23:2013–2020
Zohar S, Teramukai S, Zhou Y (2008) Bayesian design and conduct of phase II single-arm clinical trials with binary outcomes: a tutorial. Contemp Clin Trials 29:608–616
The European register of clinical trials on medicines in children, www.doc-net.org
Sempé M, Pedron G, Roy-Pernot MP, Rolland-Cachera MF (1997) Auxologie, méthode et séquences, 2nd edn. Méditions, Lyon, 205 p
Rolland-Cachera MF, Cole TJ, Sempé M, Tichet J, Rossignol C, Charraud A (1991) Body Mass Index variations: centiles from birth to 87years. Eur J Clin Nutr 45:13–21
Payen S, Zhang D, Maisin A, Popon M, Bensman A, Bouissou F, Loirat C, Gomeni R, Bressolle F, Jacqz-Aigrain E (2005) Population pharmacokinetics of mycophenolic acid in kidney transplant pediatric and adolescent patients. Ther Drug Monit 27:378–388
Choi MJ, Eustace JA, Gimenez LF, Atta MG, Scheel PJ, Sothinathan R, Briggs WA (2002) Mycophenolate mofetil treatment for primary glomerular diseases. Kidney Int 61:1098–1114
Afzal K, Bagga A, Menon S, Hari P, Jordan SC (2007) Treatment with mycophenolate mofetil and prednisolone for steroid-dependent nephrotic syndrome. Pediatr Nephrol 22:2059–2065
Weber LT, Hoecker B, Armstrong VW, Oellerich M, Tönshoff B (2008) Long-term pharmacokinetics of mycophenolic acid in pediatric renal transplant recipients over 3years posttransplant. Ther Drug Monit 30:570–575
Ghio L, Ferraresso M, Zacchello G, Murer L, Ginevri F, Belingheri M, Peruzzi L, Zanon F, Perfumo F, Berardinelli L, Tirelli S, Dello Strologo L, Fontana I, Valente U, Cardillo M, Edefonti A (2009) Longitudinal evaluation of mycophenolic acid pharmacokinetics in pediatric kidney transplant recipients, The role of post-transplant clinical and therapeutic variables. Clin Transplant 23:264–270
Zhao W, Fakhoury M, Deschênes G, Roussey G, Brochard K, Niaudet P, Tsimaratos M, André JL, Cloarec S, Cochat P, Bensman A, Azougagh S, Jacqz-Aigrain E (2010) Population pharmacokinetics and pharmacogenetics of mycophenolic acid following administration of mycophenolate mofetil in de novo pediatric renal-transplant patients. J Clin Pharmacol 50:1280–1291
Tönshoff B, David-Neto E, Ettenger R, Filler G, van Gelder T, Goebel J, Kuypers DR, Tsai E, Vinks AA, Weber LT, Zimmerhackl LB (2011) Pediatric aspects of therapeutic drug monitoring of mycophenolic acid in renal transplantation. Transpl Rev Orlando 25:78–89
Fujinaga S, Ohtomo Y, Hirano D, Nishizaki N, Someya T, Ohtsuka Y, Kaneko K, Shimizu T (2009) Mycophenolate mofetil therapy for childhood-onset steroid dependent nephrotic syndrome after long-term cyclosporine: extended experience in a single center. Clin Nephrol 72:268–273
Zhao W, Elie V, Baudouin V, Bensman A, André JL, Brochard K, Broux F, Cailliez M, Loirat C, Jacqz-Aigrain E (2010) Population pharmacokinetics and Bayesian estimator of mycophenolic acid in children with idiopathic nephrotic syndrome. Br J Clin Pharmacol 69:358–366
Saint-Marcoux F, Guigonis V, Decramer S, Gandia P, Ranchin B, Parant F, Bessenay L, Libert F, Harambat J, Bouchet S, Broux F, Compagnon P, Marquet P (2011) Development of a Bayesian estimator for the therapeutic drug monitoring of mycophenolate mofetil in children with idiopathic nephrotic syndrome. Pharmacol Res 63:423–431
Sepe V, Libetta C, Giuliano MG, Adamo G, Dal Canton A (2008) Mycophenolate mofetil in primary glomerulopathies. Kidney Int 73:154–162
Cammas B, Harambat J, Bertholet-Thomas A, Bouissou F, Morin D, Guigonis V, Bendeddouche S, Afroukh-Hacini N, Cochat P, Llanas B, Decramer S, Ranchin B (2011) Long-term effects of cyclophosphamide therapy in steroid-dependent or frequently relapsing idiopathic nephrotic syndrome. Nephrol Dial Transplant 26:178–184
Azib S, Macher MA, Kwon T, Dechartres A, Alberti C, Loirat C, Deschênes G, Baudouin V (2011) Cyclophosphamide in steroid-dependent nephrotic syndrome. Pediatr Nephrol 26:927–932
Zagury A, de Oliveira AL, de Moraes CA, de Araujo Montalvão JA, Novaes RH, de Sá VM, de Carvalho M, Dde B, Matuck T (2011) Long-term follow-up after cyclophosphamide therapy in steroid-dependent nephrotic syndrome. Pediatr Nephrol 26:915–920
MacHardy N, Miles PV, Massengill SF, Smoyer WE, Mahan JD, Greenbaum L, Massie S, Yao L, Nagaraj S, Lin JJ, Wigfall D, Trachtman H, Hu Y, Gipson DS (2009) Management patterns of childhood-onset nephrotic syndrome. Pediatr Nephrol 24:2193–2201
Gipson DS, Massengill SF, Yao L, Nagaraj S, Smoyer WE, Mahan JD, Wigfall D, Miles P, Powell L, Lin JJ, Trachtman H, Greenbaum LA (2009) Management of childhood onset nephrotic syndrome. Pediatrics 124:747–757
Manrique-Rodríguez S, Fernandez-Llamazares CM, Sanjurjo-Saez M (2010) Pharmacotherapeutic review and update of idiopathic nephrotic syndrome in children. Pharm World Sci 32:314–321
Guigonis V, Dallocchio A, Baudouin V, Dehennault M, Hachon-Le Camus C, Afanetti M, Groothoff J, Llanas B, Niaudet P, Nivet H, Raynaud N, Taque S, Ronco P, Bouissou F (2008) Rituximab treatment for severe steroid- or cyclosporine-dependent nephrotic syndrome: a multicentric series of 22 cases. Pediatr Nephrol 23:1269–1279
Sellier-Leclerc AL, Macher MA, Loirat C, Guérin V, Watier H, Peuchmaur M, Baudouin V, Deschênes G (2010) Rituximab efficiency in children with steroid-dependent nephrotic syndrome. Pediatr Nephrol 25:1109–1115
Fujinaga S, Hirano D, Nishizaki N, Someya T, Ohtomo Y, Ohtsuka Y, Shimizu T, Kaneko K (2011) Single daily high-dose mizoribine therapy for children with steroid-dependent nephrotic syndrome prior to cyclosporine administration. Pediatr Nephrol 26:479–483
Acknowledgements
We thank Wei Zhao for pharmacokinetic studies; Sarah Zohar for support for study design and Bayesian analysis. This work was supported by a research grant from the Département de la Recherche Clinique et du Développement, Assistance Publique–Hôpitaux de Paris, which was also the sponsor of the study (CRC 02 015).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baudouin, V., Alberti, C., Lapeyraque, AL. et al. Mycophenolate mofetil for steroid-dependent nephrotic syndrome: a phase II Bayesian trial. Pediatr Nephrol 27, 389–396 (2012). https://doi.org/10.1007/s00467-011-2006-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-011-2006-7