Abstract
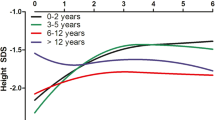
A clear definition of the appropriate growth response during recombinant human growth hormone (rhGH) treatment has never been established in the pediatric chronic kidney disease (CKD) population. We present here data from Genentech’s National Cooperative Growth Study (NCGS) on the first-year growth response in prepubertal children with CKD. Using NCGS data, we constructed response curves for the first year of rhGH therapy in 270 (186 males, 84 females) naïve-to-treatment, prepubertal children with CKD prior to transplant or dialysis. Data from both genders were combined because gender was not significantly related to height velocity (p = 0.51). Response to rhGH was expressed as height velocity (HV) in cm/year. Mean, mean ± 1SD, and mean − 2SD for HV during the first year of rhGH treatment as well as pretreatment HV were plotted versus age. Age-specific HV plots for rhGH-treated children with CKD are presented. At all ages, the first-year mean HV was greater than the mean pretreatment HV. The mean − 2SD for HV in children on rhGH treatment was similar to the mean pretreatment HV. These growth plots will be useful to clinicians for assessing a patient’s first-year growth response. We propose that a HV below the mean − 1SD is an inadequate response. These curves may help identify patients with a suboptimal growth response due to confounding medical factors and/or non-compliance.



Similar content being viewed by others
References
North American Pediatric Renal Transplant Cooperative Study (2007) Annual Report. Renal transplantation, dialysis, chronic renal insufficiency. Available at: http://spitfire.emmes.com/study/peds/resources/annlrept2007.pdf. Accessed 26 March 2008
Furth SL, Stablein D, Fine RN, Powe NR, Fivush BA (2002) Adverse clinical outcomes associated with short stature at dialysis initiation: a report of the North American Pediatric Renal Transplant Cooperative Study. Pediatrics 109:909–913
Furth SL, Hwang W, Yang C, Neu AM, Fivush BA, Powe NR (2002) Growth failure, risk of hospitalization and death for children with end-stage renal disease. Pediatr Nephrol 17:450–455
Stabler B, Clopper RR, Siegel PT, Stoppani C, Compton PG, Underwood LE (1994) Academic achievement and psychological adjustment in short children. The National Cooperative Growth Study. J Dev Behav Pediatr 15:1–6
Mahan JD, Warady BA (2006) Assessment and treatment of short stature in pediatric patients with chronic kidney disease: a consensus statement. Pediatr Nephrol 21:917–930
Fine RN, Kohaut EC, Brown D, Perlman AJ (1994) Growth after recombinant human growth hormone treatment in children with chronic renal failure: report of a multicenter randomized double-blind placebo-controlled study. Genentech Cooperative Study Group. J Pediatr 124:374–382
Bakker B, Frane J, Anhalt H, Lippe B, Rosenfeld RG (2008) Height velocity targets from the National Cooperative Growth Study for first-year growth hormone responses in short children. J Clin Endocrinol Metab 93:352–357
August GP, Lippe BM, Blethen SL, Rosenfeld RG, Seelig SA, Johanson AJ, Compton PG, Frane JW, McClellan BH, Sherman BM (1990) Growth hormone treatment in the United States: demographic and diagnostic features of 2331 children. J Pediatr 116:899–903
Cleveland W, Grosse E (1991) Computational methods for local regression. Stat Comput 1:47–52
Kuczmarski R, Ogden C, Guo S (2002) 2000 CDC growth charts for the United States: methods and development. Vital Health Stat 11:1–90
Tanner JM, Whitehouse RH, Takaishi M (1966) Standards from birth to maturity for height, weight, height velocity, and weight: British children, 1965. I. Arch Dis Child 41:454–471
Greenbaum LA, Hidalgo G, Chand D, Chiang M, Dell K, Kump T, Peschansky L, Smith HK, Boyle M, Kopf M, Metz LC, Kamel M, Mahan JD (2008) Obstacles to the prescribing of growth hormone in children with chronic kidney disease. Pediatr Nephrol 9:1531–1535
Rabkin R, Sun DF, Chen Y, Tan J, Schaefer F (2005) Growth hormone resistance in uremia, a role for impaired JAK/STAT signaling. Pediatr Nephrol 20:313–318
Roelfsema V, Clark RG (2001) The growth hormone and insulin-like growth factor axis: its manipulation for the benefit of growth disorders in renal failure. J Am Soc Nephrol 12:1297–1306
Tonshoff B, Kiepe D, Ciarmatori S (2005) Growth hormone/insulin-like growth factor system in children with chronic renal failure. Pediatr Nephrol 20:279–289
Nissel R, Lindberg A, Mehls O, Haffner D, Pfizer International Growth Database (KIGS) International Board (2008) Factors predicting the near-final height in growth hormone-treated children and adolescents with chronic kidney disease. J Clin Endocrinol Metab 93:1359–1365
Reiter EO, Price DA, Wilton P, Albertsson-Wikland K, Ranke MB (2006) Effect of growth hormone (GH) treatment on the near-final height of 1258 patients with idiopathic GH deficiency: analysis of a large international database. J Clin Endocrinol Metab 91:2047–2054
Financial disclosures
John D. Mahan, MD: Speaker’s Bureau, Genentech; Grants—Novartis, Abbott, Genentech; Bradley A. Warady, MD: Speaker’s Bureau, Genentech; James Frane, PhD: consultant for Genentech and Tercica; Ron G. Rosenfeld, MD: employed by Tercica; Rita D. Swinford, MD: Speaker’s Bureau, Genentech; Barbara Lippe, MD: employee of Genentech; D. Aaron Davis, MD: employee of Genentech.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mahan, J.D., Warady, B.A., Frane, J. et al. First-year response to rhGH therapy in children with CKD: a National Cooperative Growth Study Report. Pediatr Nephrol 25, 1125–1130 (2010). https://doi.org/10.1007/s00467-010-1450-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-010-1450-0