Abstract
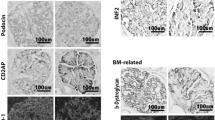
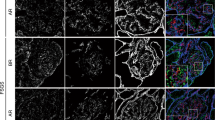
Congenital nephrotic syndrome of the Finnish type (NPHS1, CNF) is an autosomal recessive disease caused by mutations in a major podocyte protein, nephrin. NPHS1 is associated with heavy proteinuria and the development of glomerular scarring. We studied the cellular and molecular changes affecting the glomerular mesangium in NPHS1 kidneys. Marked hyperplasia of mesangial cells (MC) was mainly responsible for the early mesangial expansion in NPHS1 glomeruli. The levels of the proliferation marker, mindbomb homolog 1 and the major MC mitogen, platelet-derived growth factor, and its receptors, however, were quite normal. Only a small number of cells were positive for CD68 (marker for phagocytic cells) and CD34 (marker for mesenchymal precursor cells) in the NPHS1 mesangium. MCs strongly expressed α-smooth muscle actin, indicating myofibloblast transformation. The expression levels of the profibrotic mediators osteopontin and transforming growth factor β were up-regulated in NPHS1 glomeruli by 3.2 and 1.6-fold, respectively, compared to the controls. The synthesis by MCs of the typical fibroblast products collagen I, fibronectin, and tenascin, however, was low, and the extracellular matrix increase was caused by the accumulation of a normal MC product, collagen IV. The results indicate that severe glomerular sclerosis can develop without major qualitative cellular or molecular changes in the mesangium.


Similar content being viewed by others
References
Kestilä M, Lenkkeri U, Männikkö M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, Kashtan CE, Peltonen L, Holmberg C, Olsen A, Tryggvason K (1998) Positionally cloned gene for a novel glomerular protein-nephrin is mutated in congenital nephrotic syndrome. Mol Cell 1:575–582
Patrakka J, Kestilä M, Wartiovaara J, Ruotsalainen V, Tissari P, Lenkkeri U, Männikkö M, Visapää I, Holmberg C, Rapola J, Tryggvason K, Jalanko H (2000) Congenital nephrotic syndrome (NPHS1): features resulting from different mutations in Finnish patients. Kidney Int 58:972–980
Ruotsalainen V, Ljungberg P, Wartiovaara J, Lenkkeri U, Kestilä M, Jalanko H, Holmberg C, Tryggvason K (1999) Nephrin is specifically located at the slit diaphragm of glomerular podocytes. Proc Natl Acad Sci USA 96:7962–7967
Patrakka J, Martin P, Salonen R, Kestilä M, Ruotsalainen V, Männikkö M, Ryynänen M, Rapola J, Holmberg C, Tryggvason K, Jalanko H (2002) Proteinuria and prenatal diagnosis of congenital nephrosis in fetal carriers of nephrin gene mutations. Lancet 359:1575–1577
Kuusniemi AM, Lapatto R, Holmberg C, Karikoski R, Rapola J, Jalanko H (2005) Kidneys with heavy proteinuria shows fibrosis, inflammation and oxidative stress but no tubular phenotypic change. Kidney Int 68:121–132
Kuusniemi AM, Merenmies J, Lahdenkari AT, Holmberg C, Salmela K, Karikoski R, Rapola J, Jalanko H (2006) Glomerular sclerosis in kidneys with congenital nephrotic syndrome (NPHS1). Kidney Int 70:1423–1431
Kaukinen A, Kuusniemi A-M, Lautenschlager I, Jalanko H (2008) Glomerular endothelium in kidneys with congenital nephrosis syndrome of the Finnish type (NPHS1). Nephrol Dial Transplant 23:1224–1232
Rupprecht HD, Schöcklmann HO, Sterzel RB (1996) Cell-matrix interactions in the glomerular mesangium. Kidney Int 49:1575–1582
Moura IC, Benhamou M, Launay P, Vrtovsnik F, Blank U, Monteiro RC (2008) The glomerular response to IgA deposition in IgA nephropathy. Semin Nephrol 28:88–95
Ferrario F, Rastaldi MP (2004) Histopathological atlas of renal diseases. Membranoproliferative glomerulonephritis. J Nephrol 17:483–486
Smith DM, Fortune-Faulkner EM, Spurbeck BL (2000) Lupus nephritis: pathophysiology, diagnosis, and collaborative management. Nephrol Nurs J 27:199–204
Qian Y, Feldman E, Pennathur S, Kretzler M, Brosius FC 3rd (2008) From fibrosis to sclerosis: Mechanisms of glomerulosclerosis in diabetic nephropathy. Diabetes 57:1439–1445
El Nahas AM (2003) Plasticity of kidney: role in kidney remodelling and scarring. Kidney Int 64:1553–1563
Chebotareva NV, Proppe D, Rudolf P, Kozlovskaia LV (2002) Clinical significance of the smooth muscle actin-alpha and CD34 antigen in mesangial cells in glomerulonephritis. Ter Arkh 74:27–31
Rosenblum ND (1994) The mesangial matrix in normal and sclerotic glomerulus. Kidney Int Suppl 45:S73–S74
Yang Y, Zhang SY, Sich M, Béziau A, van den Heuvel LP, Gubler MC (2001) Glomerular extracellular matrix and growth factors in diffuse mesangial sclerosis. Pediatr Nephrol 16:429–438
Kelly DJ, Gilbert RE, Cox AJ, Soulis T, Jerums G, Cooper ME (2001) Aminoguanidine ameliorates overexpression of prosclerotic growth factors and collagen deposition in experimental diabetic nephropathy. J Am Soc Nephrol 12:2098–2107
Lenz O, Elliot SJ, Stetler-Stevenson WG (2000) Matrix metalloproteinases in renal development and disease. J Am Soc Nephrol 11:574–581
Sterzel RB, Schulze Lohoff E, Weber M, Goodman SL (1992) Interactions between glomerular mesangial cells, cytokines, and extracellular matrix. J Am Soc Nephrol 2:S126–S131
Herrera GA (2006) Plasticity of mesangial cells: A basis for understanding pathological alterations. Ultrastruct Pathol 30:471–479
Schnaper HW, Hayashida T, Hubchak SC, Poncelet AC (2003) TGF-β signal transduction and mesangial cell fibrogenesis. Am J Physiol Renal Physiol 284:F243–F252
Floege J, Burns MW, Alpers CE, Yoshimura A, Pritzl P, Gordon K, Seifert RA, Bowen-Pope DF, Couser WG, Johnson RJ (1992) Glomerular cell proliferation and PDGF expression precede glomeruloslerosis in the remnant kidney model. Kidney Int 41:297–309
Lorenzen J, Shah R, Biser A, Staicu SA, Niranjan T, Garcia AM, Gruenwald A, Thomas DB, Shatat IF, Supe K, Woroniecki RP, Susztak K (2008) The role of osteopontin in albuminuria. J Am Soc Nephrol 19:884–890
Susztak K, Böttinger E, Novetsky A, Liang D, Zhu Y, Ciccone E, Wu D, Dunn S, McCue P, Sharma K (2004) Molecular profiling of mouse kidney reveals novel genes linked to glomerular disease. Diabetes 53:784–794
Lin Y, Huang R, Chen LP, Lisoukov H, Lu ZH, Li S, Wang CC, Huang RP (2003) Profiling of cytokine expression by biotin-labeled-based protein arrays. Proteomics 3:1750–1757
Schöcklmann HO, Lang S, Sterzel RB (1999) Regulation of mesangial cell proliferation. Kidney Int 56:1199–1207
Johnson RJ, Ida H, Alpers CE, Majesky MW, Schwartz SM, Pritzi P, Gordon K, Gown AM (1991) Expression of smooth muscle cell phenotype by rat mesangial cells in immune complex nephritis: α-smooth muscle actin as a marker of mesangial cell proliferation. J Clin Invest 87:847–858
Gonlusen G, Ergin M, Paydas S, Tunali N (2001) The expression of cytoskeletal proteins (α-SMA, vimentin, desmin) in kidney tissue: a comparison of fetal, normal kidneys, and glomerulonephritis. Int Urol Nephrol 33:299–305
Stephenson LA, Haney LB, Hussaini IM, Karns LR, Glass WF 2nd (1998) Regulation of smooth muscle alpha-actin expression and hypertrophy in cultured mesangial cells. Kidney Int 54:1175–1187
Truong LD, Majesky MW, Pindur J (1994) Tenascin is synthesized and secreted by rat mesangial cells in culture and is present in extracellular matrix in human glomerular diseases. J Am Soc Nephrol 4:1771–1777
Derynck R, Zhang YE (2003) Smad-dependent and Smad-independent pathways in TGF-β family signaling. Nature 425:577–584
Lahdenkari AT, Lounatmaa K, Patrakka J, Holmberg C, Wartiovaara J, Kestilä M, Koskimies O, Jalanko H (2004) Podocytes are firmly attached to glomerular basement membrane in kidneys with heavy proteinuria. J Am Soc Nephrol 15:2611–2618
Schlöndorff D, Banas B (2009) The mesangial cell revisited: no cell is an island. J Am Soc Nephrol 20:1179–1187
Banas B, Wörnie M, Berger T, Nelson PJ, Cohen CD, Kretzler M, Pfirstinger J, Mack M, Lipp M, Gröne HJ, Schlöndorff D (2002) Roles of SLC/CCL21 and CCR7 in human kidney for mesangila proliferation, migration, apoptosis, and tissue homeostasis. J Immunol 168:4301–4307
Ahmed AK, Haylor JL, El Nahas AM, Johnson TS (2007) Localization of matrix metalloproteinases and their inhibitors in experimental progressive kidney scarring. Kidney Int 71:755–763
Hubchak SC, Runyan CE, Kreisberg JI, Schnaper HW (2003) Cytoskeletal rearrangement and signal transduction in TGF-beta1-stimulated mesangial cell collagen accumulation. J Am Soc Nephrol 14:1969–1980
Acknowledgments
We warmly thank Tuike Helmiö for her excellent technical assistance.
Confilct of Interest
The authors declare that they have no competing interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaukinen, A., Kuusniemi, AM., Helin, H. et al. Changes in glomerular mesangium in kidneys with congenital nephrotic syndrome of the Finnish type. Pediatr Nephrol 25, 867–875 (2010). https://doi.org/10.1007/s00467-009-1385-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-009-1385-5