Abstract
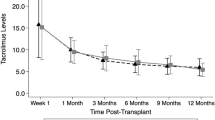
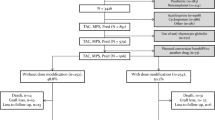
Data on the use of enteric-coated mycophenolic acid (EC-MPS) in pediatric transplantation cases are scarce. We undertook a 12-month, multicenter, open-label pilot study in which 16 de novo renal transplant patients aged 5–16 years received EC-MPS with cyclosporine A microemulsion (CsA-ME), steroids, and anti-interleukin-2 receptor antibody induction. The mean dose of EC-MPS was 916 ± 93 mg/m2 per day during weeks 1–2, 810 ± 193 mg/m2 per day during months 3–6, and 827 ± 153 mg/m2 per day during months 6–12. The mean CsA C2 level exceeded target range up to month 6 post-transplant. Efficacy failure (biopsy-proven acute rejection, graft loss, death or loss to follow-up) occurred in two patients: one patient with primary non-function underwent nephrectomy, and one patient experienced biopsy-proven acute rejection (Grade 1B, day 344) following EC-MPS dose reduction. There were no deaths. Creatinine clearance (Schwartz) was 103 ± 30 mL/min per 1.73 m2 at month 6 and 100 ± 16 mL/min per 1.73 m2 at month 12. The majority of adverse events were mild or moderate (101/126, 80.2%). In this pilot study, EC-MPS 450 mg/m2 administered twice daily with CsA, steroids, and interleukin-2 antibody induction resulted in a low rate of rejection with good renal function in a pediatric population. However, a larger, controlled trial is required to confirm these results.


Similar content being viewed by others
References
Meier-Kriesche HU, Steffen BJ, Hochberg AM, Gordon RD, Liebman MN, Morris JA, Kaplan B (2003) Long-term use of mycophenolate mofetil is associated with a reduction in the incidence and risk of late rejection. Am J Transplant 3:68–73
Halloran P, Mathew T, Tomlanovich S, Groth C, Hooftman L, Barker C (1997) Mycophenolate mofetil in renal allograft recipients: a pooled efficacy analysis of three randomized, double-blind, clinical studies in prevention of rejection. The International Mycophenolate Mofetil Renal Transplant Study Groups. Transplantation 63:39–47
Ojo AO, Meier-Kriesche HU, Hanson JA, Leichtman AB, Cibrik D, Magee JC, Wolfe RA, Agodoa LY, Kaplan B (2000) Mycophenolate mofetil reduces late renal allograft loss independent of acute rejection. Transplantation 69:2405–2409
Staskewitz A, Kirste G, Tonshoff B, Weber LT, Böswald M, Burghard R, Helmchen U, Brandis M, Zimmerhackl LB (2001) Mycophenolate mofetil in pediatric renal transplantation without induction therapy: results after 12 months of treatment. German Pediatric Renal Transplantation Study Group. Transplantation 71:638–644
Jungraithmayr T, Staskewitz A, Kirste G, Kirste G, Böswald M, Bulla M, Burghard R, Dippell J, Greiner C, Helmchen U, Klare B, Klaus G, Leichter HE, Mihatsch MJ, Michalk DV, Misselwitz J, Plank C, Querfeld U, Weber LT, Wiesel M, Tönshoff B, Zimmerhackl LB, German Pediatric Renal Transplantation Study Group (2003) Pediatric renal transplantation with mycophenolate mofetil-based immunosuppression without induction: results after three years. Transplantation 75:454–461
Hocker B, Weber LT, Bunchman T, Rashford M, Tonshoff B, Tricontinental MMF Suspension Study Group (2005) Mycophenolate mofetil suspension in pediatric renal transplantation: three-year data from the tricontinental trial. Pediatr Transplant 9:504–511
Cransberg K, Marlies Cornelissen EA, Davin JC, Van Hoeck KJ, Lilien MR, Stijnen T, Nauta J (2005) Improved outcome of pediatric kidney transplantation in the Netherlands – effect of the introduction of mycophenolate mofetil? Pediatr Transplant 9:104–111
Ferraris JR, Ghezzi LF, Vallejo G, Piantanida JJ, Araujo JL, Sojo ET (2005) Improved long-term allograft function in pediatric renal transplantation with mycophenolate mofetil. Pediatr Transplant 9:178–182
Otukesh H, Sharifian M, Basiri A, Simfroosh N, Hoseini R, Sedigh N, Golnari P, Rezai M, Fereshtenejad M (2005) Mycophenolate mofetil in pediatric renal transplantation. Transplant Proc 37:3012–3015
Arns W, Breuer S, Choudhury S, Taccard G, Lee J, Binder V, Roettele J, Schmouder R (2005) Enteric-coated mycophenolate sodium delivers bioequivalent MPA exposure compared with mycophenolate mofetil. Clin Transplant 19:199–206
Salvadori M, Holzer H, De Mattos A, Sollinger H, Arns W, Oppenheimer F, Maca J, Hall M, The ERL B301 Study Groups (2004) Enteric-coated mycophenolate sodium is therapeutically equivalent to mycophenolate mofetil in de novo renal transplant patients. Am J Transplant 4:231–236
Budde K, Curtis J, Knoll G, Chan L, Neumayer HH, Seifu Y, Hall M, ERL B302 Study Group (2004) Enteric-coated mycophenolate sodium can be safely administered in maintenance renal transplant patients: results of a 1-year study. Am J Transplant 4:237–243
Chan L, Mulgaonkar S, Walker R, Arns W, Ambühl P, Schiavelli R (2006) Patient-reported gastrointestinal symptom burden and health-related quality of life following conversion from mycophenolate mofetil to enteric-coated mycophenolate sodium. Transplantation 81:1290–1297
Behrend M (2001) Adverse gastrointestinal effects of mycophenolate mofetil: aetiology, incidence and management. Drug Saf 24:645–663
Ettenger R, Bartosh S, Choi L, Zhu W, Niederberger W, Campestrini J, Bastien MC, Schmouder R (2005) Pharmacokinetics of enteric-coated mycophenolate sodium in stable pediatric renal transplant recipients. Pediatr Transplant 9:780–787
Jacqz-Aigrain E, Shaghaghi E, Baudoin V, Popon M, Zhang D, Maisin A, Loirat C (2000) Pharmacokinetics and tolerance of mycophenolate mofetil in renal transplant children. Pediatr Nephrol 14:95–99
Garcia CD, Bittencourt VB, Tumelero A, Malheiros D, Antonello JS, Garcia VD (2006) Enteric-coated mycophenolate sodium (EC-MPS) in pediatric renal transplantation – single center experience. Transplantation 82[Suppl 3]:567
de Paula Meneses R, Kotsifas CH, Olandoski KP (2006) Conversion from mycophenolate mofetil to enteric-coated mycophenolate sodium in children with stable renal transplant. Transplantation 82[Suppl 3]:510
World Medical Association (2000) World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. Available at: https://doi.org/www.wma.net/e/policy/17-c_e.html
Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, Croker BP, Demetris AJ, Drachenberg CB, Fogo AB, Furness P, Gaber LW, Gibson IW, Glotz D, Goldberg JC, Grande J, Halloran PF, Hansen HE, Hartley B, Hayry PJ, Hill CM, Hoffman EO, Hunsicker LG, Lindblad AS, Yamaguchi Y (1999) The Banff 97 working classification of renal allograft pathology. Kidney Int 55:713–723
Schwartz GJ, Haycock GB, Edelmann CM Jr, Spitzer A (1976) A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics 58:259–263
Weber LT, Lamersdorf T, Shipkova M, Niedmann PD, Wiesel M, Zimmerhackl LB, Staskewitz A, Schütz E, Mehls O, Oellerich M, Armstrong VW, Tönshoff B (1999) Area under the plasma concentration-time curve for total, but not for free, mycophenolic acid increases in the stable phase after renal transplantation: a longitudinal study in pediatric patients. German Study Group on Mycophenolate Mofetil Therapy in Pediatric Renal Transplant Recipients. Ther Drug Monit 21:498–506
Weber LT, Shipkova M, Armstrong VW, Wagner N, Schütz E, Mehls O, Zimmerhackl LB, Oellerich M, Tönshoff B (2002) The pharmacokinetic-pharmacodynamic relationship for total and free mycophenolic Acid in pediatric renal transplant recipients: a report of the german study group on mycophenolate mofetil therapy. J Am Soc Nephrol 13:759–768
Ghio L, Ferraresso M, Viganò SM, Ginevri F, Perfumo F, Gianoglio B, Murer L, Zacchello G, Dello Strologo L, Cardillo M, Tirelli S, Valente U, Edefonti A (2005) Mycophenolate mofetil pharmacokinetic monitoring in pediatric kidney transplant recipients. Transplant Proc 37:856–858
Oellerich M, Shipkova M, Schütz E, Wieland E, Weber L, Tönshoff B, Armstrong VW (2000) Pharmacokinetic and metabolic investigations of mycophenolic acid in pediatric patients after renal transplantation: implications for therapeutic drug monitoring. German Study Group on Mycophenolate Mofetil Therapy in Pediatric Renal Transplant Recipients. Ther Drug Monit 22:20–26
Naesens M, de Loor H, Vanrenterghem Y, Kuypers DR (2007) The impact of renal allograft function on exposure and elimination of mycophenolic acid (MPA) and its metabolite MPA 7-O-glucuronide. Transplantation 84:362–373
Salvadori M, Holzer H, Civati G, Sollinger H, Lien B, Tomlanovich S, Bertoni E, Seifu Y, Marrast AC, ERL B301 Study Group on behalf of the ERL B301 Study Group (2006) Long-term administration of enteric-coated mycophenolate sodium (EC-MPS; myfortic) is safe in kidney transplant patients. Clin Nephrol 66:112–119
Budde K, Knoll G, Curtis J, Chan L, Pohanka E, Gentil M, Seifu Y, Marrast AC, Neumayer HH, ERL B302 Study Group (2006) Long-term safety and efficacy after conversion of maintenance renal transplant recipients from mycophenolate mofetil (MMF) to enteric-coated mycophenolate sodium (EC-MPS, myfortic). Clin Nephrol 66:103–111
Roberti I, Reisman L (1999) A comparative analysis of the use of mycophenolate mofetil in pediatric vs adult renal allograft recipients. Pediatr Transplant 3:231–235
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Niaudet, P., Charbit, M., Loirat, C. et al. Enteric-coated mycophenolate sodium in de novo pediatric renal transplant patients. Pediatr Nephrol 24, 395–402 (2009). https://doi.org/10.1007/s00467-008-1031-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-008-1031-7